What Are The Inner Transition Metals
Juapaving
Mar 26, 2025 · 5 min read
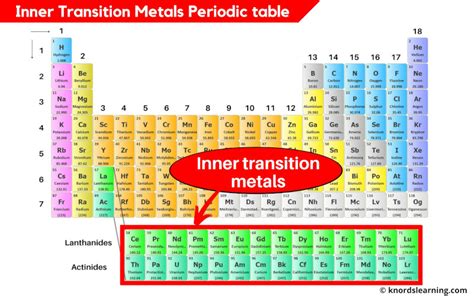
Table of Contents
What Are the Inner Transition Metals? A Deep Dive into the f-block Elements
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While many are familiar with the alkali metals, alkaline earth metals, and transition metals, a less-understood group resides within: the inner transition metals. These elements, also known as f-block elements, hold a unique position and exhibit fascinating properties that set them apart. This comprehensive article delves into the intricacies of inner transition metals, exploring their electronic configuration, properties, applications, and environmental impact.
Understanding the Electronic Configuration
The key to understanding inner transition metals lies in their electronic configuration. Unlike transition metals, which fill their d orbitals, inner transition metals fill their 4f (lanthanides) and 5f (actinides) orbitals. This filling of inner orbitals significantly influences their chemical and physical characteristics.
Lanthanides: The 4f Block
The lanthanides, also known as the rare earth elements, occupy the first inner transition series. They range from Cerium (Ce) to Lutetium (Lu), with atomic numbers 58 to 71. Their electronic configuration features the filling of the 4f orbitals, while the 6s and 5d orbitals are also involved. This subtle interplay of orbital filling leads to their similar chemical properties and makes their separation a complex undertaking.
- Key Characteristics of Lanthanides: Similar chemical properties due to similar outer electron configurations (often +3 oxidation state), high reactivity (easily oxidized), paramagnetic properties due to unpaired electrons, and characteristic spectral lines.
Actinides: The 5f Block
The actinides, from Thorium (Th) to Lawrencium (Lr), constitute the second inner transition series (atomic numbers 90 to 103). They fill their 5f orbitals, mirroring the lanthanides' 4f filling. However, a significant distinction lies in the actinides' high radioactivity. This radioactivity stems from their large nuclei and impacts their chemistry and applications considerably.
- Key Characteristics of Actinides: Radioactivity (most are radioactive isotopes), varying oxidation states (more diverse than lanthanides), higher reactivity than lanthanides, and mostly synthetic elements (beyond Uranium, Neptunium, Plutonium).
Properties of Inner Transition Metals: Similarities and Differences
While both lanthanides and actinides share the characteristic of filling their f orbitals, they exhibit both similarities and differences in their properties:
Similarities:
- Metallic Character: Both are metals exhibiting metallic luster, conductivity, and malleability.
- Paramagnetism: Many possess unpaired electrons, resulting in paramagnetic properties.
- Reactivity: Generally reactive, although their exact reactivity varies within each series.
- Formation of Complexes: They readily form coordination complexes with ligands, influencing their chemical behavior.
Differences:
- Radioactivity: Actinides are predominantly radioactive; lanthanides are significantly less radioactive or non-radioactive.
- Oxidation States: Actinides exhibit a wider range of oxidation states compared to lanthanides, which predominantly display a +3 oxidation state.
- Chemical Reactivity: Actinides are generally more reactive than lanthanides.
- Availability: Lanthanides are more readily available in the Earth's crust than actinides.
Applications of Inner Transition Metals: A Wide Spectrum of Uses
The unique properties of inner transition metals lead to a diverse range of applications across numerous fields:
Lanthanides:
- Lighting: Lanthanides are crucial in various lighting technologies, including fluorescent lamps, incandescent bulbs, and high-intensity discharge lamps. For example, Cerium is used in arc lamps, and Europium is used in red phosphors.
- Magnets: Neodymium magnets, incorporating neodymium (Nd), are exceptionally strong permanent magnets found in numerous applications, including wind turbines, hard disk drives, and electric motors.
- Catalysis: Lanthanides are used as catalysts in various chemical reactions, particularly in petroleum cracking and in the production of plastics and polymers. Cerium oxide, for instance, serves as a significant catalyst in various industries.
- Ceramics and Glasses: Lanthanides enhance the properties of various ceramics and glasses, improving their strength, color, and refractive index.
- Medical Applications: Some lanthanides, like Gadolinium, are used as contrast agents in magnetic resonance imaging (MRI).
Actinides:
- Nuclear Fuel: Uranium and Plutonium are central to nuclear power generation and nuclear weapons. Their fissile properties allow them to undergo nuclear fission, releasing vast amounts of energy.
- Nuclear Medicine: Certain isotopes of actinium, thorium, and other actinides are used in nuclear medicine for radiation therapy and diagnostic purposes.
- Scientific Research: Actinides, due to their unique properties and radioactivity, are essential for research in nuclear physics and chemistry.
- Smoke Detectors: Americium-241 is used in some ionization smoke detectors to detect smoke particles.
Environmental Impact and Ethical Considerations
The extraction, processing, and utilization of inner transition metals present both environmental and ethical challenges:
Environmental Concerns:
- Mining Impacts: The mining of rare earth elements can cause significant environmental damage, including habitat destruction, water pollution, and soil degradation.
- Radioactive Waste: The handling and disposal of radioactive actinide waste pose a long-term environmental risk due to their radioactive decay and potential for contamination.
- Toxicity: Some lanthanides and actinides can be toxic at high concentrations, impacting human health and ecosystems.
Ethical Concerns:
- Resource Scarcity: The supply of some rare earth elements is geographically concentrated, leading to geopolitical concerns and potential resource conflicts.
- Ethical Mining Practices: Concerns exist regarding ethical labor practices and environmental regulations in some regions where rare earth elements are mined.
- Nuclear Proliferation: The use of actinides in nuclear weapons raises significant ethical and security concerns regarding proliferation and potential use in warfare.
Future Directions and Research
Ongoing research aims to address the challenges associated with inner transition metals and explore their future potential:
- Sustainable Mining Practices: The development of environmentally friendly mining and processing techniques is crucial for minimizing the environmental impact of rare earth element extraction.
- Recycling and Reuse: Efforts are focused on improving the recycling and reuse of inner transition metals to reduce resource depletion and minimize waste generation.
- New Applications: Research is ongoing to discover new applications for inner transition metals in areas such as advanced materials, energy storage, and green technologies.
- Radioactive Waste Management: Improving radioactive waste management strategies is paramount for ensuring long-term environmental safety and reducing the risk of contamination.
Conclusion: The Significance of Inner Transition Metals
Inner transition metals, encompassing both lanthanides and actinides, represent a unique and fascinating group of elements with diverse properties and widespread applications. Their crucial role in modern technologies, from lighting and magnets to nuclear energy and medical applications, highlights their significance. However, responsible mining practices, efficient recycling, and careful management of radioactive waste are vital to mitigate the environmental and ethical concerns associated with their use. Continued research and innovation will be essential for ensuring the sustainable and responsible utilization of these remarkable elements in the future. Understanding their unique characteristics and potential impacts remains key to navigating the complex landscape of their use and ensuring their beneficial contribution to society while minimizing negative consequences.
Latest Posts
Latest Posts
-
Five Letter Words Ending In Ing
Mar 29, 2025
-
What Is The Freezing Point For Fahrenheit
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Are The Inner Transition Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
