A Horizontal Row In The Periodic Table Is Called
Juapaving
Mar 31, 2025 · 7 min read
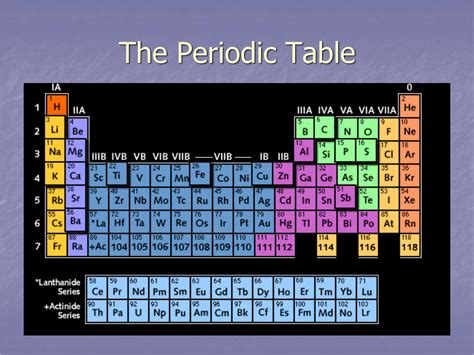
Table of Contents
A Horizontal Row in the Periodic Table is Called a Period: Understanding the Organization of Elements
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its structure is crucial to grasping the behavior of elements and their interactions. One fundamental aspect of this organization is the arrangement of elements into horizontal rows called periods. This article delves deep into the concept of periods, explaining their significance, the trends observed within them, and their connection to the electronic configuration of atoms.
What is a Period in the Periodic Table?
A period in the periodic table is a horizontal row of chemical elements. Each period represents an energy level or shell in an atom. As you move across a period from left to right, the number of electrons in the outermost shell increases. This increase in electrons directly impacts the element's properties, leading to predictable trends. There are seven periods in the standard periodic table, each characterized by specific properties and electron configurations.
The Significance of Periods
The arrangement of elements into periods isn't arbitrary; it reflects a fundamental principle of atomic structure. The number of the period corresponds to the highest principal quantum number (n) of the electrons in an element's ground state. This quantum number describes the energy level of the electrons. For instance, elements in Period 1 have electrons only in the n=1 energy level (the first shell), while elements in Period 2 have electrons in the n=1 and n=2 energy levels (the first and second shells), and so on.
This direct relationship between the period number and the principal quantum number is key to understanding the periodicity of elemental properties. The filling of electron shells dictates the chemical and physical properties of elements, and periods neatly organize elements based on these shared shell structures.
Trends Across a Period: A Detailed Exploration
As we traverse a period from left to right, several important trends in the properties of elements become apparent. These trends are directly related to the increasing number of protons and electrons within the atoms.
1. Atomic Radius: Decreasing Across a Period
Atomic radius refers to the size of an atom. Across a period, the atomic radius generally decreases. This is primarily due to the increasing nuclear charge. As the number of protons increases, the positive charge in the nucleus becomes stronger, attracting the electrons more effectively. This stronger pull draws the electrons closer to the nucleus, resulting in a smaller atomic radius.
Exceptions: While generally decreasing, there can be slight irregularities due to electron-electron repulsions and subtle variations in electron shielding.
2. Ionization Energy: Increasing Across a Period
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. This energy generally increases across a period. The increasing nuclear charge holds the electrons more tightly, making it more difficult to remove an electron. Thus, higher ionization energies are observed as you move across a period from left to right.
Exceptions: Some exceptions may occur due to the stability of half-filled or fully filled subshells.
3. Electronegativity: Increasing Across a Period
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Electronegativity generally increases across a period. The increased nuclear charge attracts the shared electrons in a bond more strongly, leading to higher electronegativity. Elements on the far right of the periodic table (excluding noble gases) have the highest electronegativity values.
Exceptions: Similar to ionization energy, exceptions exist due to electron configuration stability.
4. Electron Affinity: Generally Increasing Across a Period
Electron affinity is the change in energy when an electron is added to a neutral atom to form a negative ion. Generally, electron affinity increases across a period. The increasing nuclear charge allows the atom to more readily accept an additional electron. However, the trend isn't as consistently smooth as those observed for ionization energy or electronegativity. There are exceptions and irregularities due to the electron configurations of atoms.
5. Metallic Character: Decreasing Across a Period
Metallic character refers to the properties typically associated with metals, such as electrical conductivity, malleability, and ductility. Metallic character generally decreases across a period. As you move from left to right, elements become less metallic and more non-metallic. This is directly linked to the increase in ionization energy and electronegativity, making it harder for atoms to lose electrons and form positive ions (a characteristic of metals).
Periods and Electron Configuration: A Deeper Dive
The structure of periods is intimately connected to the electronic configuration of atoms. The elements within a period fill their outermost electron shell sequentially, following the Aufbau principle. This principle dictates the order in which electrons fill the available energy levels and sublevels.
Electron Shells and Subshells
Each period corresponds to the filling of a principal electron shell. Period 1 only fills the first shell (n=1), which consists of the 1s subshell. Period 2 fills the second shell (n=2), which includes the 2s and 2p subshells. Period 3 also fills the 3s and 3p subshells, and so on. The subsequent periods involve the filling of d and f subshells, adding complexity but maintaining the underlying principle of sequential shell filling.
The Role of Subshells
The presence of subshells explains the varying lengths of periods. The s subshell can hold up to 2 electrons, the p subshell can hold up to 6 electrons, the d subshell up to 10 electrons, and the f subshell up to 14 electrons. This explains why Period 1 is the shortest, followed by longer periods as more subshells are filled.
Transition Metals, Lanthanides, and Actinides
The longer periods (Periods 4-7) reflect the filling of d and f subshells. Periods 4 and 5 include the transition metals, whose properties are influenced by the filling of the d subshell. Periods 6 and 7 include the lanthanides and actinides, characterized by the filling of the f subshell, resulting in their distinctive chemical behaviors.
Specific Examples of Period Trends
Let's examine a specific period to illustrate the trends:
Period 3: This period starts with sodium (Na), a highly reactive alkali metal, and ends with argon (Ar), a noble gas.
- Sodium (Na): Low ionization energy, low electronegativity, readily loses an electron to form a +1 ion, exhibiting strong metallic character.
- Magnesium (Mg): Higher ionization energy and electronegativity than sodium, still metallic but less reactive.
- Aluminum (Al): Further increase in ionization energy and electronegativity, shows amphoteric properties (can act as both acid and base).
- Silicon (Si): Non-metal, higher ionization energy and electronegativity, behaves as a semiconductor.
- Phosphorus (P): Non-metal, higher ionization energy and electronegativity than silicon, exhibits various allotropes.
- Sulfur (S): Non-metal, higher ionization energy and electronegativity, forms many covalent compounds.
- Chlorine (Cl): Highly electronegative non-metal, readily gains an electron to form a -1 ion, a strong oxidizing agent.
- Argon (Ar): Noble gas, very stable and unreactive due to a full outer electron shell.
This progression clearly illustrates the trends observed across a period: decreasing atomic radius, increasing ionization energy and electronegativity, and a transition from metallic to non-metallic character.
Conclusion: The Importance of Periodicity
The organization of elements into periods is fundamental to understanding their properties and behavior. The trends observed across periods, directly linked to the electronic configuration of atoms, provide a powerful framework for predicting the chemical and physical properties of elements. By understanding the concept of periods and their relation to atomic structure, we can appreciate the elegance and utility of the periodic table, a cornerstone of modern chemistry and a testament to the organization inherent in the natural world. Understanding these trends allows chemists to predict reactivity, bonding behavior, and various other properties, making it an indispensable tool in scientific research and applications. The periodic table, with its periods and groups, is more than a chart; it is a map to understanding the elemental building blocks of our universe.
Latest Posts
Latest Posts
-
What Are The Kinds Of Motion
Apr 01, 2025
-
Describe How You Would Prepare A Supersaturated Solution
Apr 01, 2025
-
Sum Of Exterior Angles Of A Quadrilateral
Apr 01, 2025
-
When Heating A Liquid In A Test Tube You Should
Apr 01, 2025
-
How Many Bones Do Shark Have
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about A Horizontal Row In The Periodic Table Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
