How Much Electrons Does Sodium Have
Juapaving
Mar 27, 2025 · 5 min read
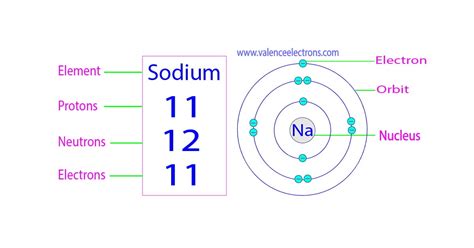
Table of Contents
How Many Electrons Does Sodium Have? A Deep Dive into Atomic Structure
Sodium, a ubiquitous element found in table salt and essential for life, is a fascinating subject for exploring the fundamentals of atomic structure. Understanding its electron configuration is key to comprehending its chemical properties and reactivity. This article delves deep into the question: How many electrons does sodium have? We'll explore the concept of atomic number, electron shells, valence electrons, and the implications of sodium's electron configuration for its behavior.
Understanding Atomic Structure: The Foundation
Before we pinpoint the number of electrons in a sodium atom, let's establish the basic framework of atomic structure. Atoms are the fundamental building blocks of matter, composed of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus.
- Neutrons: Neutrally charged particles also residing in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells.
The number of protons in an atom's nucleus defines its atomic number, which uniquely identifies the element. Crucially, in a neutral atom, the number of protons equals the number of electrons, ensuring an overall neutral charge.
Atomic Number and Sodium
Sodium's atomic number is 11. This means a neutral sodium atom contains 11 protons in its nucleus. Consequently, a neutral sodium atom also possesses 11 electrons. This fundamental fact answers the primary question of this article directly: a neutral sodium atom has eleven electrons.
Electron Shells and Subshells: Organizing the Electrons
Electrons don't orbit the nucleus randomly; they occupy specific energy levels called electron shells. These shells are arranged in increasing distance from the nucleus, with each shell capable of holding a maximum number of electrons. The shells are often designated using letters (K, L, M, N, etc.) or numbers (1, 2, 3, 4, etc.).
- Shell 1 (K): Can hold a maximum of 2 electrons.
- Shell 2 (L): Can hold a maximum of 8 electrons.
- Shell 3 (M): Can hold a maximum of 18 electrons.
- Shell 4 (N): Can hold a maximum of 32 electrons, and so on.
The electrons fill the shells in a specific order, starting with the lowest energy level (closest to the nucleus). This filling follows the Aufbau principle, which dictates that electrons occupy the lowest available energy level before filling higher energy levels. Furthermore, Hund's rule and the Pauli exclusion principle also govern electron placement within subshells.
Sodium's Electron Configuration
Applying these rules, we can determine sodium's electron configuration:
1s² 2s² 2p⁶ 3s¹
Let's break down this notation:
- 1s²: Two electrons in the 1s subshell (the lowest energy level).
- 2s²: Two electrons in the 2s subshell.
- 2p⁶: Six electrons in the 2p subshell.
- 3s¹: One electron in the 3s subshell.
This configuration confirms that sodium has a total of 11 electrons (2 + 2 + 6 + 1 = 11), distributed across three shells.
Valence Electrons: The Key to Reactivity
The outermost shell of an atom, containing the electrons most loosely bound to the nucleus, is known as the valence shell. The electrons in this shell are called valence electrons and play a crucial role in determining the atom's chemical reactivity. These electrons are readily involved in chemical bonding with other atoms.
In sodium's case, the single electron in the 3s subshell is its valence electron. This lone valence electron is relatively easily lost, making sodium highly reactive.
Sodium's Reactivity: Losing an Electron
Sodium's strong tendency to lose its single valence electron stems from its desire to achieve a stable electron configuration, similar to that of the noble gases. Noble gases possess a full outermost shell, making them chemically inert. By losing its valence electron, sodium achieves a stable octet (eight electrons) in its second shell, resembling the electron configuration of neon (1s² 2s² 2p⁶). This process forms a positively charged sodium ion (Na⁺).
This propensity to lose an electron explains sodium's reactivity, particularly with nonmetals like chlorine. When sodium reacts with chlorine, it readily donates its valence electron to chlorine, forming an ionic bond. This ionic bond leads to the formation of sodium chloride (NaCl), commonly known as table salt.
Isotopes and Electron Count: A Slight Variation
While a neutral sodium atom always has 11 electrons, it's important to consider the existence of isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. The number of neutrons affects the atom's mass but not its electron count. Therefore, different isotopes of sodium will all have 11 electrons in a neutral state.
Sodium's Role in Biology and Industry
Understanding sodium's electron configuration is essential to comprehending its crucial roles in biological systems and industrial processes. Its reactivity, dictated by its single valence electron, is fundamental to its function.
Biological Significance: Nerve Impulses and Fluid Balance
Sodium ions (Na⁺) are critical for numerous biological processes. They play a vital role in transmitting nerve impulses, maintaining fluid balance within and outside cells, and muscle contraction. The movement of sodium ions across cell membranes generates electrical signals that underpin these essential functions.
Industrial Applications: Diverse Uses
Sodium's reactivity finds wide application in diverse industrial processes. It's used in the production of various chemicals, including sodium hydroxide (NaOH), used in soap manufacturing and paper production. It's also employed in the manufacture of sodium lamps, which emit a characteristic yellow light.
Conclusion: The Significance of Eleven Electrons
The seemingly simple answer – sodium has 11 electrons – reveals a wealth of information about its atomic structure, reactivity, and importance in various contexts. Its single valence electron dictates its chemical behavior, making it a highly reactive element crucial for biological processes and numerous industrial applications. Understanding the electron configuration of sodium serves as a fundamental stepping stone to comprehending the principles of atomic structure, chemical bonding, and the behavior of elements. The number eleven, therefore, isn't just a simple numerical value; it's a key to unlocking a deeper understanding of the world around us.
Latest Posts
Latest Posts
-
5 Letter Words Starting With A P
Mar 30, 2025
-
How Many Hundreds Are In 7000
Mar 30, 2025
-
Write 72 As A Product Of Prime Factors
Mar 30, 2025
-
How To Find The Latus Rectum Of A Parabola
Mar 30, 2025
-
Greatest Common Factor Of 24 And 30
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Much Electrons Does Sodium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
