Write The Electron Configuration For A Neutral Atom Of Magnesium
Juapaving
Mar 28, 2025 · 5 min read
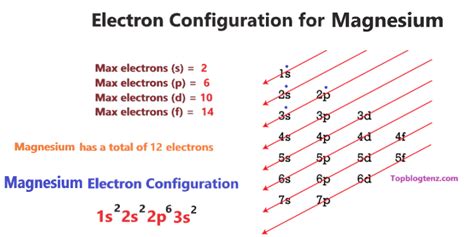
Table of Contents
Unveiling the Electron Configuration of Magnesium: A Deep Dive
Magnesium, a silvery-white alkaline earth metal, plays a crucial role in numerous biological and industrial processes. Understanding its electron configuration is fundamental to grasping its chemical behavior and reactivity. This comprehensive guide will not only explain the electron configuration of a neutral magnesium atom but will also explore the underlying principles of electron configuration, delve into the periodic table's role in predicting it, and illustrate its implications for magnesium's chemical properties.
Understanding Electron Configuration
The electron configuration of an atom describes the arrangement of electrons in its electron shells and subshells. This arrangement dictates how an atom interacts with other atoms, forming chemical bonds and influencing its overall properties. It follows specific rules based on quantum mechanics, which govern the behavior of electrons within an atom.
The Aufbau Principle and Hund's Rule
Two key principles guide the electron filling of orbitals:
-
The Aufbau Principle: Electrons fill the lowest energy levels first. This means that electrons occupy orbitals starting from the lowest energy level (1s) and progressively move to higher energy levels (2s, 2p, 3s, 3p, and so on) until all electrons are assigned.
-
Hund's Rule: Within a subshell (e.g., 2p), electrons individually occupy each orbital within that subshell before pairing up. This maximizes the total spin of the electrons in the subshell, leading to greater stability. Each orbital can hold a maximum of two electrons with opposite spins (represented by ↑ and ↓).
Orbital Notation and Electron Configuration Notation
Electron configurations can be represented in two ways:
-
Orbital Notation: This uses boxes to represent orbitals, with arrows indicating electrons. This visually demonstrates Hund's rule.
-
Electron Configuration Notation: This uses a shorthand notation where the principal quantum number (n), the subshell (s, p, d, or f), and the number of electrons in that subshell are specified. For example, 1s² means two electrons in the 1s subshell.
Determining Magnesium's Electron Configuration
Magnesium (Mg) has an atomic number of 12, meaning it has 12 protons and, in a neutral atom, 12 electrons. To determine its electron configuration, we follow the Aufbau principle and Hund's rule:
-
1s²: The first two electrons fill the lowest energy level, the 1s subshell.
-
2s²: The next two electrons fill the 2s subshell.
-
2p⁶: The next six electrons fill the 2p subshell, with each of the three 2p orbitals receiving two electrons each (following Hund's rule).
-
3s²: The remaining two electrons fill the 3s subshell.
Therefore, the complete electron configuration of a neutral magnesium atom is 1s²2s²2p⁶3s².
Visualizing Magnesium's Electron Configuration
Using orbital notation, we can visualize the electron arrangement as follows:
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑↓
3s: ↑↓
Magnesium's Position in the Periodic Table and its Electron Configuration
Magnesium's position in the periodic table directly reflects its electron configuration. It belongs to Group 2 (alkaline earth metals) and Period 3.
-
Group 2: The group number indicates the number of valence electrons—the electrons in the outermost shell. Magnesium has two valence electrons in its 3s subshell. This explains its +2 oxidation state, readily losing these two electrons to achieve a stable noble gas configuration (like Neon).
-
Period 3: The period number indicates the highest principal quantum number (n) of the occupied electron shell. Magnesium's highest occupied shell is n=3.
Implications of Magnesium's Electron Configuration for its Chemical Properties
Magnesium's electron configuration dictates its chemical behavior:
-
Reactivity: Magnesium's two valence electrons are relatively loosely held. This makes it highly reactive, readily losing these electrons to form Mg²⁺ ions and achieving a stable noble gas configuration (isoelectronic with Neon). This explains its strong reducing ability.
-
Bonding: Magnesium typically forms ionic bonds with nonmetals, transferring its two valence electrons to achieve a stable octet. For example, it reacts vigorously with oxygen to form magnesium oxide (MgO), and with chlorine to form magnesium chloride (MgCl₂).
-
Metallic Properties: Magnesium exhibits metallic properties due to the delocalization of its valence electrons. These electrons are not tightly bound to individual atoms and are free to move throughout the metal lattice, resulting in good electrical and thermal conductivity, malleability, and ductility.
-
Biological Role: The +2 cation form of magnesium plays an essential role in numerous biological processes. It acts as a cofactor for many enzymes, is vital for muscle contraction, nerve impulse transmission, and DNA replication.
Variations in Electron Configuration: Excited States
While the configuration 1s²2s²2p⁶3s² represents magnesium in its ground state (lowest energy level), it can exist in excited states when an electron absorbs energy and jumps to a higher energy level. For example, one 3s electron might be excited to a higher 3p orbital. These excited states are less stable and will return to the ground state through emitting the absorbed energy as light or heat.
Isoelectronic Species
Species with the same number of electrons exhibit similar chemical properties. Magnesium, in its +2 ionic form (Mg²⁺), is isoelectronic with Neon (Ne), both having 10 electrons. This explains why Mg²⁺ is relatively unreactive compared to neutral magnesium.
Conclusion
The electron configuration of magnesium, 1s²2s²2p⁶3s², is a cornerstone to understanding its chemical behavior and properties. Its two valence electrons explain its reactivity, ionic bonding tendencies, metallic properties, and significant biological functions. Understanding electron configurations is not only crucial for studying magnesium but for predicting and interpreting the properties of all elements in the periodic table. By applying the fundamental principles of the Aufbau principle and Hund's rule, we can accurately predict and visualize the electron configuration of any element, providing a vital foundation for comprehending the intricacies of chemistry. Further exploration into quantum mechanics provides even deeper insights into the complex behavior of electrons within an atom, solidifying our understanding of the periodic table and the properties of elements.
Latest Posts
Latest Posts
-
The Temperature At Which A Solid Becomes A Liquid
Mar 31, 2025
-
How Many Pairs Of Homologous Chromosomes Do Females Have
Mar 31, 2025
-
54 As Product Of Prime Factors
Mar 31, 2025
-
Organelles That Are The Sites Of Protein Synthesis
Mar 31, 2025
-
What Is The Percentage Of 2 5
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Write The Electron Configuration For A Neutral Atom Of Magnesium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
