Why Doesn't Hydrogen Have A Neutron
Juapaving
Mar 28, 2025 · 6 min read
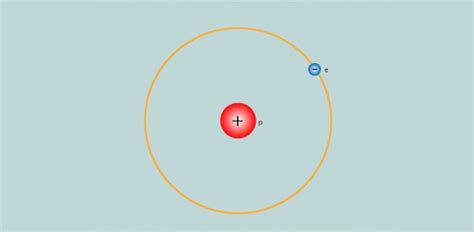
Table of Contents
Why Doesn't Hydrogen Have a Neutron? Unraveling the Mystery of the Simplest Atom
Hydrogen, the most abundant element in the universe, stands apart from other elements due to its unique atomic structure. Unlike most atoms, the most common isotope of hydrogen, protium, possesses only a single proton and a single electron, lacking a neutron entirely. This seemingly simple fact holds profound implications for our understanding of atomic structure, nuclear physics, and the evolution of the universe. This article delves deep into the reasons behind hydrogen's neutron-less existence, exploring the underlying physics and the consequences of this unique atomic configuration.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we delve into the specifics of hydrogen, it's crucial to grasp the fundamental building blocks of atoms: protons, neutrons, and electrons. These subatomic particles define an atom's properties and behavior.
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons determines an element's atomic number and its identity on the periodic table. Hydrogen, with its atomic number of 1, has one proton.
-
Neutrons: Neutrally charged particles also found in the nucleus. Neutrons contribute to an atom's mass but don't affect its charge. Their presence is crucial for nuclear stability in heavier elements.
-
Electrons: Negatively charged particles orbiting the nucleus. The number of electrons typically equals the number of protons in a neutral atom, maintaining electrical neutrality. Electrons determine an atom's chemical properties and its interactions with other atoms.
Isotopes of Hydrogen: A Closer Look
While the most common form of hydrogen (protium, ¹H) lacks a neutron, hydrogen actually exists in three naturally occurring isotopes:
-
Protium (¹H): The most abundant isotope, consisting of one proton and one electron. It lacks a neutron.
-
Deuterium (²H or D): Contains one proton, one neutron, and one electron. It's a stable isotope, though significantly less abundant than protium.
-
Tritium (³H or T): Contains one proton, two neutrons, and one electron. It's a radioactive isotope with a relatively short half-life.
The absence of a neutron in protium isn't a matter of chance; it's a consequence of the fundamental forces governing the nucleus. The strong nuclear force, which binds protons and neutrons together, is crucial for nuclear stability. However, this force has a limited range, and the electromagnetic repulsion between positively charged protons plays a significant role, especially in lighter nuclei.
The Role of the Strong Nuclear Force and Electromagnetic Repulsion
The stability of an atomic nucleus depends on a delicate balance between the strong nuclear force and the electromagnetic force. The strong nuclear force, as its name suggests, is a very strong attractive force acting between nucleons (protons and neutrons). This force overcomes the electromagnetic repulsion between protons, which tends to push them apart.
In the case of protium (¹H), the presence of only one proton eliminates the need for a neutron to counteract electromagnetic repulsion. There's simply no significant repulsive force to overcome. The strong nuclear force is sufficient to bind the single proton, and the nucleus remains stable.
However, as we move to heavier elements, the number of protons increases, leading to a stronger electromagnetic repulsion. Neutrons become increasingly essential to provide additional strong nuclear force attraction without adding to the repulsive electromagnetic force. They act as a kind of "nuclear glue," stabilizing the nucleus and preventing it from breaking apart.
The Significance of Neutron-Proton Ratio in Nuclear Stability
The ratio of neutrons to protons (N/Z ratio) plays a crucial role in nuclear stability. For lighter elements, a N/Z ratio close to 1 is usually sufficient for stability. Protium, with a N/Z ratio of 0, still maintains stability because of the low number of protons and the absence of repulsive forces.
As the atomic number increases, a higher N/Z ratio is needed for stability. This is because the increasing number of protons requires a greater number of neutrons to counterbalance the electromagnetic repulsion. This explains why heavier elements require more neutrons than protons to maintain a stable nucleus. The isotopes of hydrogen showcase this trend—protium has no neutrons, deuterium has an equal number of protons and neutrons, and tritium has more neutrons than protons. The instability of tritium, due to its high neutron-to-proton ratio, further illustrates the importance of this delicate balance.
The Abundance of Protium: A Cosmic Perspective
The prevalence of protium in the universe is a direct consequence of its stability and the conditions prevailing during the Big Bang. The Big Bang nucleosynthesis, which occurred in the early universe, produced primarily hydrogen and helium. The extreme temperatures and densities during this period favored the formation of protium, the simplest and most stable form of hydrogen.
The abundance of protium is also linked to the ease with which protons can be formed. Protons are fundamental particles, while neutrons are composed of quarks, making proton formation simpler and more energetically favorable under the conditions of the early universe. Consequently, protium, with its single proton, emerged as the dominant form of hydrogen.
Hydrogen's Unique Properties: Implications for Chemistry and Physics
The absence of a neutron in protium significantly impacts hydrogen's chemical and physical properties. Its small size and single electron make it highly reactive, readily forming covalent bonds with other atoms. Its low mass contributes to its high diffusivity and unique behavior in various physical processes. In fusion reactions, protium's simplicity enables relatively straightforward fusion processes, unlike heavier isotopes or elements. These properties have profound consequences across various scientific fields, including astrophysics, chemistry, and energy research.
Beyond Protium: The Roles of Deuterium and Tritium
While protium dominates, deuterium and tritium play significant roles in different contexts:
-
Deuterium: Used as a tracer in various scientific studies, and it is considered a potential fuel for future fusion reactors.
-
Tritium: Used in certain types of nuclear weapons and in self-powered lighting devices. Its radioactivity necessitates careful handling.
Conclusion: A Simple Atom with Profound Implications
The seemingly simple question of why hydrogen lacks a neutron in its most common isotope leads us to a deeper understanding of atomic structure, nuclear physics, and the evolution of the universe. The absence of a neutron in protium is not an accident but a consequence of the interplay between the strong nuclear force and electromagnetic repulsion. This unique atomic configuration has profound implications for hydrogen's properties and its abundance in the universe. The study of hydrogen isotopes provides invaluable insights into the fundamental forces governing the universe and offers potential pathways for future energy technologies. The seemingly simple hydrogen atom, therefore, reveals a wealth of complexity, providing a rich area of scientific inquiry that continues to fascinate and inspire researchers.
Latest Posts
Latest Posts
-
Lcm Of 9 And 12 And 15
Mar 31, 2025
-
Which Of The Following Processes Returns Carbon To The Atmosphere
Mar 31, 2025
-
5 Letter Word That Starts With Vi
Mar 31, 2025
-
Can Acquired Characteristics Be Passed On The Next Generation
Mar 31, 2025
-
What Are The Three Body Parts Of A Mollusk
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Why Doesn't Hydrogen Have A Neutron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
