Why Are Noble Gasses Not Reactive
Juapaving
Mar 14, 2025 · 5 min read
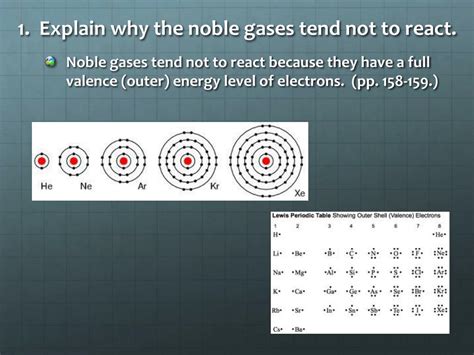
Table of Contents
Why Are Noble Gases Not Reactive? Unraveling the Mystery of Inertness
The noble gases, also known as inert gases, occupy Group 18 of the periodic table. They're a fascinating group of elements, renowned for their extreme unreactivity. This lack of reactivity, a defining characteristic, stems from their unique electronic configurations. Understanding why noble gases are so unreactive requires delving into the fundamentals of atomic structure and chemical bonding. This article will explore the reasons behind this inertness, examining their electronic structure, the significance of stable octets, and exceptions to their general unreactivity.
The Electronic Structure: A Key to Inertness
The key to understanding the unreactivity of noble gases lies in their electronic structure. Each noble gas atom, except for helium, possesses a full outermost electron shell, also known as the valence shell. This full valence shell, typically containing eight electrons (an octet), represents a state of exceptional stability.
-
Helium (He): Helium, with only two electrons, has a completely filled 1s orbital. This duplet configuration provides exceptional stability, analogous to the octet rule for other noble gases.
-
Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn): These elements possess eight electrons in their outermost shells, achieving a stable octet configuration.
This stable electronic arrangement makes noble gases incredibly resistant to gaining, losing, or sharing electrons with other atoms. Chemical reactions, fundamentally, involve the rearrangement of electrons to achieve a more stable electronic configuration. Since noble gases already possess this stable configuration, they exhibit minimal tendency to participate in such rearrangements.
The Significance of the Octet Rule
The octet rule, a cornerstone of chemical bonding theory, states that atoms tend to gain, lose, or share electrons to achieve a full valence shell of eight electrons. Noble gases, already possessing this stable octet (or duplet for helium), naturally resist any changes that would disrupt this stable arrangement. The energy required to disrupt this stable configuration is significantly high, making reactions with noble gases highly improbable under normal conditions.
Why Achieving a Stable Octet is Crucial
The drive towards a stable electron configuration is rooted in the principles of quantum mechanics. Electrons occupy specific energy levels and orbitals within an atom. A full valence shell represents a lower energy state than an incomplete one. Atoms undergo reactions to reach the lowest possible energy state, enhancing their stability. Noble gases, having already attained this low-energy, stable state, show no inclination to participate in reactions that would increase their energy.
Electrostatic Interactions and Noble Gas Stability
Beyond the octet rule, the electrostatic interactions within the noble gas atoms contribute to their inertness. The positively charged nucleus is effectively shielded from external interactions by the negatively charged electrons in the filled valence shell. This shielding effect makes it difficult for other atoms to interact with the noble gas atom’s electrons, reducing the likelihood of chemical bonding.
Exceptions to the Rule: The Reactivity of Xenon and Radon
While noble gases are generally unreactive, it's crucial to acknowledge that xenon (Xe) and, to a lesser extent, radon (Rn), have shown some evidence of reactivity under specific, highly energetic conditions. This is because these heavier noble gases have larger atomic radii and weaker nuclear attraction on their outer electrons. This makes it slightly easier, although still energetically unfavorable, to perturb their electronic configuration.
Reactions involving xenon and radon are often facilitated by highly reactive species or under extreme conditions such as high pressure and low temperatures. These reactions typically involve highly electronegative elements such as fluorine and oxygen. Even then, these reactions are rare and require specialized conditions.
Examples of Xenon compounds include Xenon hexafluoride (XeF₆) and Xenon tetrafluoride (XeF₄). These compounds were synthesized under specific high-energy conditions and represent exceptions to the general rule of noble gas inertness.
Applications of Noble Gases Leveraging their Inertness
The unreactive nature of noble gases makes them exceptionally useful in various applications where inertness is crucial. Here are some notable examples:
-
Lighting: Noble gases are used in lighting applications due to their ability to emit light when electrically excited. Neon signs are a classic example of this application. Argon is also used in incandescent light bulbs.
-
Welding: Argon's inertness protects the weld zone from oxidation during welding processes.
-
Medical applications: Helium is used in MRI machines and for cryogenic purposes. Argon is used in laser treatments.
-
Aerospace: Helium, being lightweight and non-reactive, is used in balloons and airships.
-
Protecting Reactive Materials: Noble gases provide an inert atmosphere to prevent oxidation and contamination of sensitive materials.
The Future of Noble Gas Research
Despite their reputation for inertness, research into the reactivity of noble gases continues. Scientists are exploring ways to synthesize new compounds and explore the limits of their reactivity under even more extreme conditions. Further research in this area could lead to novel applications and a deeper understanding of chemical bonding.
Conclusion
The unreactivity of noble gases is a direct consequence of their complete valence electron shells. This stable electronic configuration, whether it's a duplet (helium) or an octet (other noble gases), represents a low-energy state that resists disruption. While some heavier noble gases exhibit limited reactivity under specialized conditions, their overall inertness remains a defining feature, contributing significantly to their unique applications in various fields. The inherent stability of noble gases makes them crucial elements in numerous industries, demonstrating the important role of electronic configuration in determining the chemical behavior of elements. Further research will undoubtedly continue to refine our understanding of these fascinating elements and potentially uncover even more unexpected properties.
Latest Posts
Latest Posts
-
What Type Of Bond Holds Nitrogen Bases Together
Mar 14, 2025
-
Which Of The Following Are Examples Of Plasmas
Mar 14, 2025
-
Is Slime A Non Newtonian Fluid
Mar 14, 2025
-
What Type Of Triangle Has Two Equal Sides
Mar 14, 2025
-
What Is The Function Of Areolar Tissue
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Why Are Noble Gasses Not Reactive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
