Why Are Ionic Compounds Soluble In Water
Juapaving
Mar 25, 2025 · 6 min read
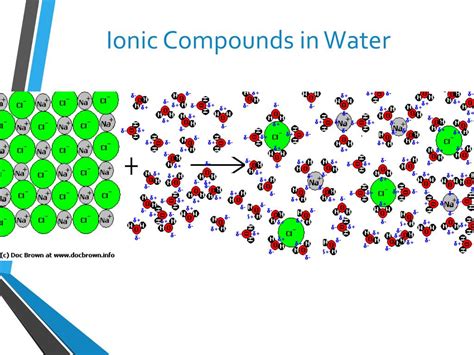
Table of Contents
Why Are Ionic Compounds Soluble in Water? A Deep Dive into Polarity and Hydration
The solubility of ionic compounds in water is a fundamental concept in chemistry with far-reaching implications in various fields, from biology and geology to industrial processes and environmental science. Understanding why some ionic compounds readily dissolve in water while others remain stubbornly insoluble requires a close examination of the interplay between molecular forces and the properties of both the solute and the solvent. This comprehensive article will delve into the intricate details of this process, exploring the key factors that govern the solubility of ionic compounds in water.
The Polar Nature of Water: The Key to Dissolution
Water, the universal solvent, possesses a unique molecular structure that underpins its exceptional ability to dissolve a wide range of substances, particularly ionic compounds. The oxygen atom in a water molecule (H₂O) is more electronegative than the hydrogen atoms, resulting in an uneven distribution of charge. This means that the oxygen atom carries a partial negative charge (δ-), while the hydrogen atoms carry partial positive charges (δ+). This uneven charge distribution makes water a polar molecule. This polarity is crucial for its interaction with ionic compounds.
Understanding Polarity and its Impact
The polarity of water allows it to effectively interact with the charged ions that constitute ionic compounds. Remember that ionic compounds are formed through the electrostatic attraction between positively charged cations and negatively charged anions. The strength of this attraction determines the lattice energy of the ionic compound – a measure of the energy required to break apart the crystal lattice structure.
The Hydration Process: Surrounding Ions with Water Molecules
When an ionic compound is added to water, the polar water molecules are attracted to the charged ions. This interaction is called hydration. The negatively charged oxygen atoms in water molecules surround the positively charged cations, while the positively charged hydrogen atoms surround the negatively charged anions. This process effectively shields the ions from each other, weakening the electrostatic forces that hold the ionic lattice together.
The Role of Hydration Energy
The energy released during hydration is called hydration energy. It is the energy released when water molecules surround ions, forming a hydration shell. For an ionic compound to dissolve, the hydration energy must be greater than the lattice energy. If the hydration energy is insufficient to overcome the lattice energy, the ionic compound will remain insoluble.
Factors Affecting the Solubility of Ionic Compounds in Water
Several factors influence the extent to which an ionic compound dissolves in water. These factors are intricately related and often work in concert to determine the overall solubility:
1. Lattice Energy: The Strength of the Ionic Bond
As mentioned earlier, lattice energy is a crucial factor. High lattice energy implies strong electrostatic forces within the crystal lattice, making it difficult for water molecules to break apart the structure. Compounds with high lattice energies, typically those with small, highly charged ions, tend to be less soluble. For example, magnesium oxide (MgO) has a very high lattice energy and is practically insoluble in water.
2. Hydration Energy: The Strength of Ion-Dipole Interactions
The magnitude of hydration energy is directly proportional to the charge density of the ions. Higher charge density means stronger ion-dipole interactions with water molecules, leading to greater hydration energy and increased solubility. Smaller ions with larger charges have higher charge densities and thus stronger hydration. For instance, smaller ions like lithium (Li⁺) have higher hydration energies compared to larger ions like potassium (K⁺).
3. Ion Size: Balancing Attraction and Repulsion
The size of the ions also plays a critical role. Larger ions have lower charge densities, resulting in weaker ion-dipole interactions and lower hydration energy. This makes them less soluble than smaller ions with the same charge. The increased size also leads to weaker electrostatic attraction between the ions in the crystal lattice, partially offsetting the effect of lower hydration energy.
4. Charge of Ions: The Power of Electrostatic Forces
The magnitude of the charge on the ions is directly proportional to the strength of both the lattice energy and the hydration energy. Higher charges lead to stronger electrostatic attractions, both within the crystal lattice and between the ions and water molecules. However, the net effect on solubility depends on the balance between these two opposing forces. Compounds with highly charged ions often exhibit lower solubility due to very high lattice energies that are difficult to overcome.
5. Temperature: The Effect of Kinetic Energy
Temperature affects the solubility of many ionic compounds. Increasing the temperature generally increases the kinetic energy of both the water molecules and the ions. This increased kinetic energy helps to overcome the lattice energy, leading to increased solubility. However, the effect of temperature on solubility is not always straightforward and can vary depending on the specific compound.
Examples Illustrating Solubility Trends
Let's consider some specific examples to solidify our understanding:
-
Sodium chloride (NaCl): NaCl is highly soluble in water. While it has a reasonably strong lattice energy, the hydration energy of its relatively small ions (Na⁺ and Cl⁻) is sufficiently large to overcome the lattice energy.
-
Silver chloride (AgCl): AgCl is virtually insoluble in water. Its lattice energy is exceptionally high due to the strong electrostatic attraction between Ag⁺ and Cl⁻ ions. The hydration energy, while significant, is not enough to overcome the substantial lattice energy.
-
Potassium nitrate (KNO₃): KNO₃ is highly soluble in water. The relatively large size of K⁺ and NO₃⁻ ions results in a lower lattice energy compared to NaCl. The hydration energy is still substantial, leading to good solubility.
-
Calcium carbonate (CaCO₃): CaCO₃ is sparingly soluble in water. The high charge on Ca²⁺ and the relatively large size of CO₃²⁻ contribute to a moderate lattice energy and hydration energy, resulting in limited solubility.
Beyond the Basics: Factors Influencing Solubility beyond Simple Hydration
While the concepts of lattice energy and hydration energy provide a robust framework for understanding ionic solubility, other factors can also play a significant role:
-
Common Ion Effect: The presence of a common ion in solution can significantly decrease the solubility of an ionic compound. For instance, adding NaCl to a saturated solution of AgCl will decrease the solubility of AgCl due to the common ion effect.
-
Complex Ion Formation: Certain ions can form complex ions with water molecules or other ligands, altering their charge density and thus affecting their solubility. The formation of complex ions can either increase or decrease solubility depending on the specific circumstances.
-
pH: The pH of the solution can influence the solubility of ionic compounds, particularly those that are amphoteric or contain weak acids or bases. Changes in pH can alter the charge of the ions, affecting their hydration energy and solubility.
-
Solvent Effects: While this article focuses on water, the solubility of ionic compounds is also dependent on the properties of the solvent. Polar solvents generally dissolve ionic compounds more readily than non-polar solvents.
Conclusion: A Holistic Perspective on Ionic Solubility
The solubility of ionic compounds in water is a complex phenomenon governed by a delicate balance of various factors. While lattice energy and hydration energy are primary determinants, other factors such as ion size, charge, temperature, the common ion effect, complex ion formation, pH, and the nature of the solvent contribute to the overall solubility. A comprehensive understanding of these interconnected factors is essential for predicting and manipulating the solubility of ionic compounds in a variety of applications. This detailed analysis helps us appreciate the multifaceted nature of chemical interactions and their profound implications across scientific disciplines. By understanding these fundamentals, we can better predict and control chemical reactions and processes, opening doors for innovations in many fields.
Latest Posts
Latest Posts
-
What Is The Function Of The Filament
Mar 26, 2025
-
Which Of The Following Statements Are Correct
Mar 26, 2025
-
Least Common Multiple Of 2 And 9
Mar 26, 2025
-
Is 54 A Prime Number Or A Composite Number
Mar 26, 2025
-
Unit Of Measurement For Specific Heat Capacity
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Why Are Ionic Compounds Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
