Whoch Of The Following Has The Units Og G/mol
Juapaving
Mar 04, 2025 · 5 min read
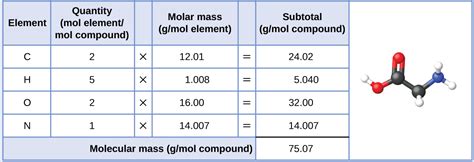
Table of Contents
Which of the Following Has the Units of g/mol? Understanding Molar Mass
The unit g/mol, or grams per mole, is a fundamental unit in chemistry representing molar mass. Understanding molar mass is crucial for numerous chemical calculations and concepts. This comprehensive guide will delve into what molar mass is, why it uses the units g/mol, how to calculate it, and differentiate it from other related chemical concepts. We'll also explore examples to solidify your understanding.
What is Molar Mass?
Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry representing Avogadro's number (approximately 6.022 x 10²³) of entities, whether those entities are atoms, molecules, ions, or formula units. Therefore, molar mass essentially tells us the mass of 6.022 x 10²³ particles of a particular substance.
Think of it like this: if you have a dozen eggs, you have 12 eggs. Similarly, if you have one mole of carbon atoms, you have 6.022 x 10²³ carbon atoms. The molar mass tells you the total mass of that massive collection of atoms.
The units of molar mass are grams per mole (g/mol) because it represents the mass (in grams) per one mole of the substance.
Calculating Molar Mass: A Step-by-Step Guide
Calculating the molar mass of a substance depends on its chemical formula.
For elements: The molar mass of an element is numerically equal to its atomic mass (found on the periodic table). The units are g/mol.
- Example: The atomic mass of carbon (C) is approximately 12.01. Therefore, the molar mass of carbon is 12.01 g/mol. This means one mole of carbon atoms weighs 12.01 grams.
For compounds: To calculate the molar mass of a compound, you need to add up the molar masses of all the atoms present in its chemical formula.
- Example: Let's calculate the molar mass of water (H₂O).
- The molar mass of hydrogen (H) is approximately 1.01 g/mol.
- The molar mass of oxygen (O) is approximately 16.00 g/mol.
- Water has two hydrogen atoms and one oxygen atom.
- Therefore, the molar mass of H₂O is: (2 x 1.01 g/mol) + (1 x 16.00 g/mol) = 18.02 g/mol.
For ionic compounds: The process is similar to calculating the molar mass of covalent compounds. You simply add up the molar masses of all the ions present in the formula unit.
- Example: Let's calculate the molar mass of sodium chloride (NaCl).
- The molar mass of sodium (Na) is approximately 22.99 g/mol.
- The molar mass of chlorine (Cl) is approximately 35.45 g/mol.
- Therefore, the molar mass of NaCl is: 22.99 g/mol + 35.45 g/mol = 58.44 g/mol.
Distinguishing Molar Mass from Other Concepts
It's crucial to differentiate molar mass from other related concepts that might use similar units but represent different properties:
-
Molecular Weight (Molecular Mass): While often used interchangeably with molar mass, molecular weight refers to the mass of a single molecule. The units are often expressed as amu (atomic mass units) or Da (Dalton), rather than g/mol. However, numerically, it's the same value as the molar mass.
-
Formula Weight (Formula Mass): This term is used for ionic compounds and refers to the mass of one formula unit. Similar to molecular weight, the numerical value is identical to molar mass, but the context and potentially units might differ.
-
Gram Atomic Mass: This term specifically refers to the mass of one mole of atoms of a particular element. It's equivalent to the molar mass of the element.
-
Gram Molecular Mass: This refers to the mass of one mole of molecules of a compound. It is equivalent to the molar mass of the compound.
The key distinction lies in the scale: molar mass deals with a macroscopic amount (one mole), while molecular/formula weight deals with a single entity at the microscopic level. However, the numerical values are the same and the units are often used interchangeably in practical applications.
Why is Molar Mass Important?
Molar mass is a cornerstone of many chemical calculations, including:
-
Stoichiometry: It allows us to convert between mass and moles, essential for balancing chemical equations and determining the amounts of reactants and products in a reaction.
-
Solution Chemistry: It's crucial for calculating solution concentrations (molarity, molality) and determining the osmotic pressure of solutions.
-
Gas Laws: Molar mass is involved in calculations related to the ideal gas law, relating the volume, pressure, temperature, and amount of a gas.
-
Thermochemistry: Molar mass is used in calculating enthalpy changes, entropy changes, and Gibbs free energy changes in chemical reactions.
-
Titrations: Molar mass is used extensively in calculating the concentrations of acids and bases and other analytes.
Practical Applications and Examples
Let's illustrate the importance of molar mass with some examples:
Example 1: Stoichiometry
Consider the reaction: 2H₂ + O₂ → 2H₂O
If we have 10 grams of hydrogen gas (H₂), how many grams of water (H₂O) can we produce?
-
Calculate moles of H₂: Molar mass of H₂ = 2.02 g/mol. Moles of H₂ = 10 g / 2.02 g/mol = 4.95 moles.
-
Use stoichiometry: From the balanced equation, 2 moles of H₂ produce 2 moles of H₂O. Therefore, 4.95 moles of H₂ produce 4.95 moles of H₂O.
-
Calculate mass of H₂O: Molar mass of H₂O = 18.02 g/mol. Mass of H₂O = 4.95 moles x 18.02 g/mol = 89.19 grams.
Example 2: Solution Chemistry
How many grams of sodium chloride (NaCl) are needed to prepare 500 mL of a 0.1 M solution?
-
Calculate moles of NaCl: Molarity (M) = moles/volume (in liters). 0.1 M = moles / 0.5 L. Moles of NaCl = 0.05 moles.
-
Calculate mass of NaCl: Molar mass of NaCl = 58.44 g/mol. Mass of NaCl = 0.05 moles x 58.44 g/mol = 2.92 grams.
These examples highlight how molar mass acts as a bridge between the macroscopic world (grams) and the microscopic world (moles), enabling precise quantitative calculations in chemistry.
Conclusion
Molar mass, with its units of g/mol, is a fundamental concept in chemistry. Understanding its definition, calculation, and applications is essential for mastering various chemical concepts and performing quantitative analyses. By grasping the relationship between molar mass, moles, and Avogadro's number, you can confidently tackle stoichiometry problems, solution chemistry calculations, and numerous other chemical applications. Remember to always refer to a periodic table for accurate atomic masses when calculating molar masses.
Latest Posts
Latest Posts
-
Least Common Multiple For 4 And 7
Mar 04, 2025
-
What Is The Roman Numeral For 30
Mar 04, 2025
-
Which Point On The Number Line Represents
Mar 04, 2025
-
What Is The Least Common Multiple Of 10 And 7
Mar 04, 2025
-
How Many Chambers Does A Frog Heart Have
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Whoch Of The Following Has The Units Og G/mol . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
