Which Statement Best Describes The Law Of Conservation Of Mass
Juapaving
Mar 23, 2025 · 5 min read
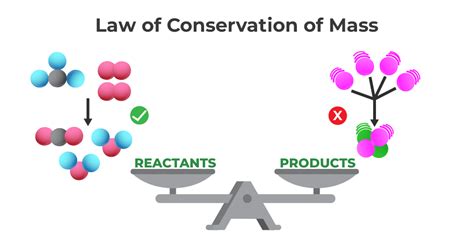
Table of Contents
Which Statement Best Describes the Law of Conservation of Mass?
The Law of Conservation of Mass, a cornerstone of chemistry and physics, dictates that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. Understanding this fundamental principle is crucial for comprehending a wide array of scientific phenomena, from simple chemical reactions to the complexities of nuclear processes. This article delves deep into the law, exploring various statements that attempt to describe it, identifying the most accurate one, and examining its implications and exceptions.
Understanding the Core Principle
Before we delve into the nuances of different statements, let's solidify our understanding of the core concept. The law essentially states that in any closed system, the total mass remains constant, regardless of any changes that occur within that system. This means that during a chemical reaction, for instance, the total mass of the reactants (the starting materials) will always equal the total mass of the products (the resulting substances). No matter how complex the reaction, the mass remains conserved.
This isn't to say that the form of the mass remains unchanged. Chemical reactions involve the rearrangement of atoms, forming new molecules with different properties. However, the total number of atoms, and therefore the total mass, remains consistent. Similarly, physical changes, such as melting ice, do not alter the total mass; the mass of the ice remains the same as the mass of the water it becomes.
Evaluating Different Statements Describing the Law
Several statements attempt to encapsulate the Law of Conservation of Mass. Let's analyze some of them, identifying their strengths and weaknesses:
Statement 1: "Matter cannot be created or destroyed."
This statement, while a common simplification, is not entirely accurate. While it captures the essence of the law in many everyday scenarios, it fails to account for situations involving nuclear reactions. In nuclear reactions, a small amount of mass can be converted into energy (and vice versa) according to Einstein's famous equation, E=mc². Therefore, this statement is too broad and lacks the precision needed for a complete description.
Statement 2: "The total mass of reactants equals the total mass of products in a chemical reaction."
This statement is significantly more accurate than the first. It correctly focuses on chemical reactions, where the law is most directly applicable. It emphasizes the crucial equality between the mass of the starting materials and the mass of the resulting substances. This is a precise description within the context of chemical transformations. However, it doesn't encompass physical changes.
Statement 3: "Mass is conserved in a closed system."
This statement is the most accurate and comprehensive of the three. It explicitly includes the crucial concept of a "closed system," a system where no mass can enter or leave. This is vital because the law only holds true in such isolated systems. If mass can be added or removed, the total mass will naturally change, and the law will not apply. The statement also encompasses both chemical and physical changes, eliminating the limitations of Statement 2.
Statement 4: "The total amount of matter remains constant in a closed system."
This statement, like Statement 3, acknowledges the importance of a closed system. However, the term "matter" can be ambiguous. While it generally refers to mass, the inclusion of this slightly broader term may introduce unnecessary complexities. Statement 3, focusing explicitly on "mass," is arguably more precise and unambiguous.
Implications and Applications of the Law
The Law of Conservation of Mass has far-reaching implications across various scientific fields. It forms the basis of stoichiometry, allowing chemists to accurately predict the amounts of reactants and products in chemical reactions. This is essential for industrial processes, pharmaceutical production, and numerous other applications.
The law is also crucial in environmental science. Understanding mass balance helps us track pollutants, manage resources, and assess the environmental impact of various activities. For instance, monitoring the mass of pollutants entering and leaving a lake allows for accurate assessment of water quality.
Furthermore, the law plays a significant role in geology. Studying the mass balance of geological processes, such as erosion and sedimentation, helps us understand the evolution of landscapes and the Earth's systems.
Exceptions and Refinements
While the Law of Conservation of Mass holds true in the vast majority of situations, it does have some exceptions. As mentioned earlier, nuclear reactions represent a significant deviation from the law. In nuclear reactions, a small amount of mass is converted into energy, or vice versa, altering the total mass slightly. However, even in these cases, the total energy and mass combined remain conserved, reflecting the broader principle of mass-energy conservation.
Conclusion: Choosing the Best Statement
After analyzing the various statements, it's clear that Statement 3, "Mass is conserved in a closed system," is the most accurate and comprehensive description of the Law of Conservation of Mass. It's precise, unambiguous, and encompasses all relevant aspects of the law, including both chemical and physical changes while highlighting the crucial condition of a closed system. While simplified statements might be useful for introductory explanations, a thorough understanding requires acknowledging the specific conditions and potential exceptions, making Statement 3 the most robust and scientifically accurate representation. This understanding is fundamental to mastering chemistry, physics, and related fields.
The law, while seemingly simple, underpins a vast array of scientific principles and applications. From balancing chemical equations to predicting the outcome of complex reactions, the principle of mass conservation continues to be an indispensable tool in scientific inquiry and technological advancement. The nuanced understanding of this fundamental law, including its limitations and refinements, allows for a deeper appreciation of the interconnectedness of matter and energy within our universe. A thorough grasp of the law is paramount for anyone seeking a solid foundation in scientific understanding.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 10 And 2
Mar 25, 2025
-
Least Common Multiple 16 And 24
Mar 25, 2025
-
Which Items Influence The Trial Balance Agreement
Mar 25, 2025
-
How Much Is 40 Inches In Feet
Mar 25, 2025
-
The Name Of The Si Unit For Force Is The
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Statement Best Describes The Law Of Conservation Of Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
