Which One Of The Following Is An Ionic Compound
Juapaving
Mar 23, 2025 · 5 min read
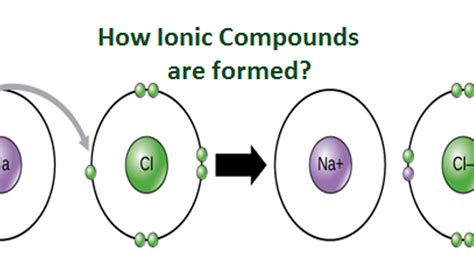
Table of Contents
Which One of the Following is an Ionic Compound? Understanding Chemical Bonding
Determining whether a compound is ionic or covalent is a fundamental concept in chemistry. Understanding the difference hinges on the nature of the chemical bond holding the atoms together. This article will delve into the intricacies of ionic bonding, contrasting it with covalent bonding, and providing a robust framework for identifying ionic compounds. We'll also tackle common misconceptions and explore advanced scenarios.
What is an Ionic Compound?
An ionic compound is formed through the electrostatic attraction between oppositely charged ions. This attraction arises from the transfer of one or more electrons from a metal atom to a nonmetal atom. The metal atom loses electrons, becoming a positively charged cation, while the nonmetal atom gains electrons, becoming a negatively charged anion. The strong electrostatic force between these oppositely charged ions constitutes the ionic bond.
Key Characteristics of Ionic Compounds:
- High Melting and Boiling Points: The strong electrostatic forces between ions require significant energy to overcome, resulting in high melting and boiling points.
- Crystalline Structure: Ionic compounds typically form a well-ordered crystalline structure, with ions arranged in a regular, repeating pattern. This structure maximizes electrostatic attraction and minimizes repulsion.
- Hardness and Brittleness: While generally hard, ionic crystals are brittle. Applying stress can misalign the ions, leading to strong repulsive forces and causing the crystal to fracture.
- Solubility in Water: Many ionic compounds are soluble in water, as the polar water molecules can effectively surround and separate the ions.
- Electrical Conductivity: Ionic compounds conduct electricity when molten (liquid) or dissolved in water, as the mobile ions can carry an electric current. In solid form, they are typically insulators because the ions are fixed in place.
Understanding the Difference Between Ionic and Covalent Bonds
To confidently identify an ionic compound, it's crucial to understand the contrasting nature of covalent bonds. In a covalent bond, atoms share electrons rather than transferring them. This sharing creates a stable molecule held together by the shared electron pair. Covalent bonds typically occur between nonmetal atoms.
Here's a table summarizing the key differences:
| Feature | Ionic Bond | Covalent Bond |
|---|---|---|
| Bond Formation | Electron transfer | Electron sharing |
| Participating Atoms | Metal and nonmetal | Nonmetals |
| Electronegativity Difference | Large | Small or zero |
| Melting/Boiling Point | High | Relatively low |
| Solubility in Water | Often soluble | Variable, often insoluble |
| Electrical Conductivity | Conducts when molten or dissolved | Generally does not conduct |
| Structure | Crystalline | Molecular (discrete molecules) |
Identifying Ionic Compounds: Practical Strategies
Let's explore practical strategies for identifying ionic compounds, focusing on factors that predict the type of bonding:
1. Electronegativity Difference:
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. A large difference in electronegativity between two atoms strongly suggests an ionic bond. Generally, a difference of 1.7 or greater on the Pauling scale is indicative of an ionic bond.
2. Periodic Table Trends:
The periodic table provides valuable insights. Ionic compounds typically involve a metal (located on the left side of the table) and a nonmetal (located on the right side, excluding noble gases). The further apart the elements are on the periodic table, the more likely the bond will be ionic.
3. Recognizing Common Ions:
Familiarity with common ions and their charges is essential. For instance, alkali metals (Group 1) typically form +1 ions, alkaline earth metals (Group 2) form +2 ions, halogens (Group 17) form -1 ions, and so on. Recognizing these common ions can quickly help identify ionic compounds.
4. Analyzing Chemical Formulas:
The chemical formula itself can provide clues. Ionic compounds typically consist of a cation followed by an anion. The formula is written in a way that the overall charge is neutral (the positive charges from cations balance the negative charges from anions). For example, NaCl (sodium chloride) shows a +1 sodium ion (Na⁺) and a -1 chloride ion (Cl⁻), resulting in a neutral compound.
Addressing Common Misconceptions
Several misconceptions surround ionic compounds. Let's clarify these:
- Not all metal-nonmetal combinations are ionic: While many are, some compounds formed between a metal and a nonmetal might exhibit significant covalent character. This is particularly true when the metal is a transition metal or the electronegativity difference is relatively small.
- Polar covalent bonds are not ionic: Polar covalent bonds involve unequal sharing of electrons, resulting in a partial positive and partial negative charge within the molecule. While they possess some ionic character, they are fundamentally different from ionic bonds where electrons are fully transferred.
- All ionic compounds are crystalline solids at room temperature: This is mostly true, but some ionic compounds can exist in other phases under specific conditions.
Advanced Scenarios and Exceptions
The rules for identifying ionic compounds are not always absolute. Some compounds exhibit characteristics of both ionic and covalent bonding, creating a spectrum rather than a clear-cut distinction. These are often referred to as polar covalent compounds with significant ionic character.
Examples of Complex Cases:
- Transition Metal Compounds: Transition metals often form compounds with varying oxidation states, leading to complexities in predicting bond type.
- Compounds involving Polyatomic Ions: Polyatomic ions, such as sulfate (SO₄²⁻) or nitrate (NO₃⁻), add another layer of complexity to determining the overall ionic character of a compound.
- Coordinate Covalent Bonds: In these bonds, both electrons in the shared pair originate from the same atom. While these bonds can be present in ionic compounds, they don't negate the overall ionic character if a significant electronegativity difference exists.
Conclusion: Mastering Ionic Compound Identification
Identifying an ionic compound requires a holistic understanding of chemical bonding principles. By combining knowledge of electronegativity differences, periodic table trends, common ions, and chemical formulas, you can develop a robust ability to distinguish ionic compounds from covalent ones. Remember that while guidelines exist, exceptions and borderline cases do arise, underscoring the nuanced nature of chemical bonding. This article aims to build your foundational understanding, allowing you to approach complex scenarios with confidence and accuracy. Consistent practice and engagement with diverse examples will further hone your skills in recognizing and characterizing ionic compounds.
Latest Posts
Latest Posts
-
What Are The Factors Of 200
Mar 25, 2025
-
5 Letter Words End With Er
Mar 25, 2025
-
How Many Cm Is 14 Inches
Mar 25, 2025
-
How Many Feet In A 100 Yards
Mar 25, 2025
-
Difference Between Nuclear Reaction And Chemical Reaction
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which One Of The Following Is An Ionic Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
