Which One Of The Following Compound Is Aromatic
Juapaving
Mar 28, 2025 · 6 min read
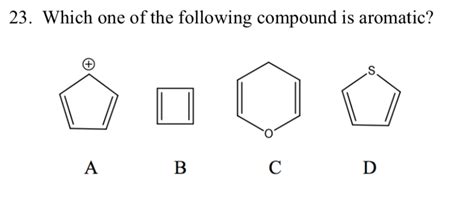
Table of Contents
Delving into Aromaticity: Which Compound Reigns Supreme?
Aromaticity, a fascinating concept in organic chemistry, dictates the exceptional stability and reactivity of certain cyclic compounds. Understanding the rules governing aromaticity is crucial for predicting the behavior of molecules and designing new materials. This article delves deep into the criteria for aromaticity, exploring various examples and ultimately determining which of a hypothetical set of compounds exhibits aromatic characteristics. We will analyze several examples, highlighting the importance of each criterion for aromaticity.
What Makes a Compound Aromatic? The Huckel's Rule and Beyond
The defining characteristic of an aromatic compound lies in its adherence to Hückel's rule. This rule states that a planar, cyclic, conjugated molecule will be aromatic if it contains a total of (4n + 2) π electrons, where 'n' is a non-negative integer (0, 1, 2, 3, and so on). This magic number of π electrons allows for delocalization of the electrons throughout the ring, creating a highly stable system.
But Hückel's rule isn't the whole story. Aromatic compounds must also fulfill three crucial conditions:
-
Planarity: The molecule must be able to adopt a planar conformation, allowing for continuous overlap of p-orbitals involved in the π system. Any significant deviation from planarity disrupts this crucial overlap, hindering aromaticity.
-
Cyclic: The conjugated π system must be part of a continuous ring structure. Open-chain conjugated systems, while exhibiting some degree of delocalization, do not possess the unique stability of aromatic compounds.
-
Conjugation: The molecule must have a continuous system of overlapping p-orbitals. This means that each atom in the ring must contribute one p-orbital to the delocalized π electron system. This continuous overlap allows for the electrons to be spread across the entire ring.
Analyzing Hypothetical Compounds: A Case Study
Let's now consider a set of hypothetical compounds and analyze them based on these criteria to determine their aromaticity:
Compound 1: Cyclobutadiene (C₄H₄)
Cyclobutadiene is a four-membered ring with alternating single and double bonds. It appears to fulfill the cyclic and conjugated criteria. However, let's examine its adherence to Hückel's rule:
- Number of π electrons: 4 (two double bonds, each contributing two π electrons).
- Hückel's rule: 4n + 2 = 4 => n = ½ (this is not a whole number)
Since cyclobutadiene doesn't satisfy Hückel's rule, it's not aromatic. Furthermore, its square planar structure is prone to significant distortions to alleviate the ring strain and electron repulsion.
Compound 2: Benzene (C₆H₆)
Benzene, the quintessential aromatic compound, perfectly exemplifies the criteria for aromaticity:
- Planarity: The six-membered ring is planar.
- Cyclic: It possesses a cyclic structure.
- Conjugation: Six p-orbitals from the six carbon atoms overlap to form a continuous π system.
- Hückel's rule: 6 π electrons (three double bonds). 4n + 2 = 6 => n = 1 (a whole number).
Benzene thus fulfills all conditions and is definitively aromatic. Its exceptional stability is a direct consequence of this aromaticity.
Compound 3: Cyclooctatetraene (C₈H₈)
Cyclooctatetraene is an eight-membered ring with alternating single and double bonds. While cyclic and seemingly conjugated, it presents a crucial deviation:
- Planarity: It adopts a non-planar tub-shaped conformation to minimize strain. This breaks the continuous overlap of p-orbitals, preventing significant delocalization.
- Hückel's rule: 8 π electrons. 4n + 2 = 8 => n = 1.5 (not a whole number)
Even if it were planar, the failure to satisfy Hückel's rule would negate aromaticity. Cyclooctatetraene is therefore non-aromatic.
Compound 4: Pyridine (C₅H₅N)
Pyridine is a six-membered ring containing five carbon atoms and one nitrogen atom. The nitrogen atom contributes one electron to the π system:
- Planarity: The ring is planar.
- Cyclic: It's a cyclic compound.
- Conjugation: Six p-orbitals contribute to the π system (five from carbon, one from nitrogen).
- Hückel's rule: 6 π electrons. 4n + 2 = 6 => n = 1.
Pyridine satisfies all criteria and is therefore aromatic. The presence of the nitrogen atom does not disrupt the aromaticity; it actively participates in the delocalized π system.
Compound 5: Cyclopentadienyl Anion (C₅H₅⁻)
The cyclopentadienyl anion is a five-membered ring with alternating single and double bonds. However, the negative charge is crucial:
- Planarity: The ring is planar.
- Cyclic: It's a cyclic compound.
- Conjugation: Five p-orbitals contribute, along with the lone pair on the negatively charged carbon.
- Hückel's rule: 6 π electrons (five from the double bonds and one from the lone pair). 4n + 2 = 6 => n = 1.
This anion satisfies all criteria and is therefore aromatic. The extra electron from the negative charge contributes to achieving the magic number of 6 π electrons.
Compound 6: Furan (C₄H₄O)
Furan is a five-membered ring containing four carbon atoms and one oxygen atom. The oxygen atom's lone pair participates in the π system.
- Planarity: The ring is planar.
- Cyclic: It’s a cyclic structure.
- Conjugation: Six electrons participate in the delocalized π system (four from carbons, two from oxygen's lone pair).
- Hückel's Rule: 6 π electrons. 4n + 2 = 6 => n = 1
Furan satisfies all criteria and is therefore aromatic.
Compound 7: Pyrrole (C₄H₅N)
Similar to furan, pyrrole is a five-membered ring with four carbon atoms and one nitrogen atom. The nitrogen atom’s lone pair participates in the π system.
- Planarity: The ring is planar.
- Cyclic: It’s a cyclic structure.
- Conjugation: Six electrons participate in the delocalized π system (four from carbons, two from nitrogen’s lone pair).
- Hückel's Rule: 6 π electrons. 4n + 2 = 6 => n = 1
Pyrrole also satisfies all criteria and is therefore aromatic.
Compound 8: Thiophene (C₄H₄S)
Thiophene, similar to furan and pyrrole, is a five-membered ring with a sulfur atom. The sulfur atom's lone pair participates in the π system.
- Planarity: The ring is planar.
- Cyclic: It’s a cyclic structure.
- Conjugation: Six electrons participate in the delocalized π system (four from carbons, two from sulfur's lone pair).
- Hückel's Rule: 6 π electrons. 4n + 2 = 6 => n = 1
Thiophene is therefore aromatic.
Conclusion: Recognizing Aromatic Compounds
Determining aromaticity requires a meticulous evaluation of all criteria. A compound must be planar, cyclic, conjugated, and satisfy Hückel's rule with (4n + 2) π electrons. While Hückel's rule serves as a powerful predictor, deviations from planarity can significantly affect a compound's aromaticity. This detailed analysis highlights the subtle interplay of structural features that ultimately determine a molecule's aromatic character. Understanding these principles provides a powerful tool for predicting the properties and reactivity of organic molecules, paving the way for the design and synthesis of new compounds with desired characteristics. The examples provided illustrate the breadth of aromatic systems, encompassing not only hydrocarbons but also heterocycles containing atoms like nitrogen, oxygen, and sulfur, thereby broadening the scope of this crucial concept in organic chemistry.
Latest Posts
Latest Posts
-
From A Gas To A Liquid
Mar 31, 2025
-
How Many Feet Is 16 Meters
Mar 31, 2025
-
Examples Of Gas To A Liquid
Mar 31, 2025
-
Horizontal Cross Section Of A Cone
Mar 31, 2025
-
Land That Is Suitable For Growing Crops
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Which One Of The Following Compound Is Aromatic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
