Which Of The Following Is A Unit Of Energy
Juapaving
Mar 29, 2025 · 6 min read
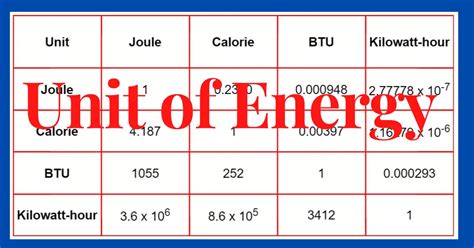
Table of Contents
Which of the following is a unit of energy? A Comprehensive Guide
The question, "Which of the following is a unit of energy?" might seem simple at first glance. However, understanding the nuances of energy units requires delving into the fundamental concepts of energy itself and the various systems used to measure it. This comprehensive guide will explore the different units of energy, their relationships, and the contexts in which they're most commonly used. We'll also delve into related concepts like work, power, and the various forms of energy.
Understanding Energy: A Fundamental Concept
Before diving into the units, let's establish a firm understanding of energy. Energy, in its simplest form, is the capacity to do work. Work, in physics, is defined as the force applied to an object multiplied by the distance over which that force is applied. This means that energy is inherently linked to the ability to cause change or motion.
Energy exists in various forms, including:
- Kinetic Energy: The energy of motion. A moving car, a flying bird, and even the molecules vibrating within a substance all possess kinetic energy.
- Potential Energy: Stored energy. This includes gravitational potential energy (an object's height above the ground), elastic potential energy (a stretched spring), and chemical potential energy (stored within chemical bonds).
- Thermal Energy (Heat): The energy associated with the temperature of a substance. Higher temperatures indicate greater thermal energy.
- Electrical Energy: The energy associated with the flow of electric charge.
- Radiant Energy (Light): Energy that travels in the form of electromagnetic waves.
- Nuclear Energy: Energy stored within the nucleus of an atom. This is released during nuclear reactions such as fission and fusion.
Common Units of Energy
Energy is measured in a variety of units, depending on the context and the system of units being used. Some of the most common units include:
-
Joule (J): The International System of Units (SI) unit of energy. One joule is defined as the work done when a force of one newton is applied over a distance of one meter. It's a versatile unit used across various scientific disciplines.
-
Calorie (cal): A unit of energy commonly used in the context of food and nutrition. One calorie is the amount of energy required to raise the temperature of one gram of water by one degree Celsius. Note that the "calorie" used in food labeling (kcal or Calorie) is actually a kilocalorie (1 kcal = 1000 cal).
-
British Thermal Unit (BTU): A unit of energy commonly used in the United States to express heating and cooling capacity. One BTU is defined as the amount of heat required to raise the temperature of one pound of water by one degree Fahrenheit.
-
Electronvolt (eV): A unit of energy commonly used in atomic and nuclear physics. One electronvolt is the energy gained by a single electron when it moves through a potential difference of one volt. It's particularly useful for describing the energy levels of atoms and the energy of particles in accelerators.
-
Kilowatt-hour (kWh): A unit of energy commonly used in the context of electricity consumption. One kilowatt-hour is the energy consumed by a device with a power of one kilowatt operating for one hour. This is the unit you see on your electricity bill.
-
Erg: A smaller unit of energy in the CGS (centimeter-gram-second) system.
Relationships Between Energy Units
These units are not independent; they are all interchangeable through conversion factors. For example:
- 1 calorie (cal) ≈ 4.184 joules (J)
- 1 BTU ≈ 1055 joules (J)
- 1 kWh = 3.6 x 10⁶ joules (J)
- 1 eV ≈ 1.602 x 10⁻¹⁹ joules (J)
Choosing the Right Unit
The choice of energy unit depends on the specific application. For instance:
-
Joules (J) are the preferred unit in most scientific contexts due to their place within the SI system. They are versatile and readily used in calculations involving work, kinetic energy, potential energy, and many other forms of energy.
-
Calories (cal) and kilocalories (kcal) are commonly used for measuring the energy content of food. The nutritional information on food packaging usually uses kcal (often written as "Calorie" with a capital "C").
-
BTUs are frequently employed in the heating, ventilation, and air conditioning (HVAC) industry to express the heating and cooling capacity of equipment.
-
kWh is the standard unit for billing electricity consumption to consumers. It represents the total energy used over a period of time.
-
Electronvolts (eV) are essential in fields like particle physics and nuclear physics due to their convenience in dealing with microscopic energy levels and particle interactions.
Energy, Work, and Power: Interrelated Concepts
It's crucial to distinguish between energy, work, and power. While intimately related, they represent different physical quantities:
-
Energy: The capacity to do work. It's a scalar quantity (only has magnitude).
-
Work: The transfer of energy that results from a force acting on an object and causing a displacement. It's also a scalar quantity.
-
Power: The rate at which work is done or energy is transferred. It's a scalar quantity expressed as work done or energy transferred per unit time. The SI unit of power is the watt (W), which is equal to one joule per second (J/s).
Real-World Applications of Energy Units
Understanding energy units is crucial in various aspects of daily life and various industries:
-
Electricity Bills: Understanding kilowatt-hours (kWh) is vital for managing household electricity costs.
-
Nutrition: Calories (kcal) are essential for tracking dietary intake and maintaining a healthy lifestyle.
-
Engineering: Joules (J), BTUs, and kWh are vital in engineering designs for calculating energy efficiency, power requirements, and energy consumption of various systems.
-
Physics and Chemistry: Joules (J) and electronvolts (eV) are essential for performing calculations and understanding the behavior of matter at the atomic and subatomic levels.
-
Climate Change: Measuring energy output from various sources, such as fossil fuels, is crucial for understanding and mitigating climate change.
Conclusion
The question of which unit measures energy is not a simple one-size-fits-all answer. The correct unit depends entirely on the context. From the fundamental joule used across scientific disciplines to the practical kilowatt-hour used for billing electricity and the calorie used in nutrition, understanding the relationship between these different units is paramount. This comprehensive overview has provided a deep dive into energy units, their applications, and their interrelation with concepts like work and power. By understanding these concepts, individuals can navigate a wide range of applications that involve energy and energy transfer, from daily routines to advanced scientific research. The next time you encounter the question of which unit measures energy, you'll be equipped with the knowledge to provide a comprehensive and insightful response.
Latest Posts
Latest Posts
-
What Is The Difference Between Place And Value
Apr 01, 2025
-
What Is 15 Percent Of 200
Apr 01, 2025
-
How Tall Is 155 Cm In Feet
Apr 01, 2025
-
44 Rounded To The Nearest Ten
Apr 01, 2025
-
How To Find Ph Given Pka
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Unit Of Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
