Which Of The Following Is A Triprotic Acid
Juapaving
Mar 29, 2025 · 5 min read
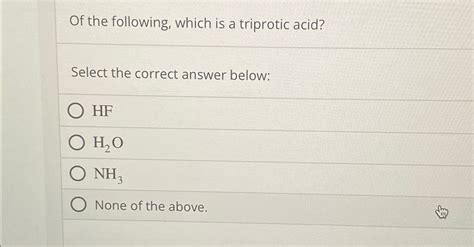
Table of Contents
Which of the Following is a Triprotic Acid? Understanding Polyprotic Acids
The question, "Which of the following is a triprotic acid?" hinges on understanding the concept of polyprotic acids. This article delves deep into the world of acids, focusing specifically on triprotic acids and how they differ from monoprotic and diprotic acids. We'll explore the definition, examples, and practical applications of these important chemical compounds. We'll also touch upon the implications of their stepwise ionization in various chemical reactions and solutions.
Understanding Acids and Their Classification
Before diving into triprotic acids, let's establish a foundation. An acid, in the simplest terms, is a substance that donates a proton (H⁺ ion) to another substance, a process known as protonation. This donation process increases the concentration of hydronium ions (H₃O⁺) in aqueous solutions, leading to a decrease in pH, making the solution acidic.
Acids are classified based on the number of protons they can donate per molecule. This classification is crucial in determining their behavior in chemical reactions and solutions:
-
Monoprotic Acids: These acids can donate only one proton per molecule. Examples include hydrochloric acid (HCl), nitric acid (HNO₃), and acetic acid (CH₃COOH).
-
Diprotic Acids: These acids can donate two protons per molecule. Sulfuric acid (H₂SO₄) and carbonic acid (H₂CO₃) are classic examples. Their ionization occurs in two steps, each with its own acid dissociation constant (Ka).
-
Triprotic Acids: This is the focus of our discussion. Triprotic acids can donate three protons per molecule. Their ionization is a three-step process, each with its own Ka value.
-
Polyprotic Acids: This is a broader term encompassing all acids capable of donating more than one proton per molecule. Diprotic and triprotic acids fall under this category.
Identifying Triprotic Acids: Key Characteristics
The defining characteristic of a triprotic acid is its ability to undergo three successive ionization steps. Let's illustrate this with a common example: phosphoric acid (H₃PO₄).
Phosphoric acid (H₃PO₄) ionization:
- First Ionization: H₃PO₄ + H₂O ⇌ H₂PO₄⁻ + H₃O⁺ (Ka1)
- Second Ionization: H₂PO₄⁻ + H₂O ⇌ HPO₄²⁻ + H₃O⁺ (Ka2)
- Third Ionization: HPO₄²⁻ + H₂O ⇌ PO₄³⁻ + H₃O⁺ (Ka3)
Notice that each ionization step produces a different conjugate base. The Ka values (acid dissociation constants) represent the extent of ionization for each step. Generally, Ka1 > Ka2 > Ka3, meaning the first ionization is the most significant. This stepwise ionization is a key feature differentiating triprotic acids from other acid types.
Examples of Triprotic Acids
While phosphoric acid is the most well-known example, several other compounds exhibit triprotic acid behavior. Here are a few:
-
Citric Acid (C₆H₈O₇): Found naturally in citrus fruits, citric acid plays a vital role in biological processes and is widely used as a food additive and cleaning agent. Its three carboxyl groups (-COOH) are responsible for its triprotic nature.
-
Arsenic Acid (H₃AsO₄): This inorganic acid is analogous to phosphoric acid in structure and behavior. It’s significantly more toxic than phosphoric acid and should be handled with extreme care.
-
Phosphorous Acid (H₃PO₃): This is a bit of an exception. While it has three hydrogen atoms, only two are acidic. It's considered a diprotic acid, not a triprotic acid. The third hydrogen is bonded directly to the phosphorus atom and does not ionize readily.
Differentiating Triprotic from Other Acids
To confidently identify a triprotic acid, consider these points:
-
Chemical Formula: Look for a formula with three acidic hydrogens (H) that are readily ionizable. However, simply counting hydrogens isn't enough. As shown with phosphorous acid, structural considerations are crucial.
-
Stepwise Ionization: Triprotic acids undergo three distinct ionization steps, each characterized by a different Ka value. This stepwise ionization is reflected in titration curves.
-
Titration Curve: A titration curve of a triprotic acid will reveal three distinct equivalence points, corresponding to the three ionization steps. Each equivalence point marks the complete neutralization of one proton.
-
Conductivity: Aqueous solutions of triprotic acids will exhibit relatively high electrical conductivity due to the presence of multiple ions formed during ionization.
Applications of Triprotic Acids
Triprotic acids, especially phosphoric acid and citric acid, have extensive applications across various industries:
-
Phosphoric Acid: Widely used in fertilizers, food additives (e.g., as an acidulant in sodas), and detergents. Its ability to form salts with various metals is also exploited in industrial processes.
-
Citric Acid: Its applications span food and beverage industries (flavoring, preservation), pharmaceuticals (as a buffering agent), and cleaning products (chelating agent). Its biodegradability and relatively low toxicity contribute to its widespread use.
-
Other Triprotic Acids: Arsenic acid, although highly toxic, has found niche uses in pesticides (historically) and wood preservatives. However, its toxicity limits its applications.
The Importance of Ka Values in Understanding Triprotic Acid Behavior
The three Ka values (Ka1, Ka2, Ka3) provide critical insights into the relative strength of each ionization step. They determine the concentration of each ionic species (H₃A, H₂A⁻, HA²⁻, A³⁻) present in a solution at equilibrium. The significant difference between the Ka values indicates that the successive ionizations are progressively less favorable, in line with the increased negative charge on the conjugate base. This is also reflected in the pH of a triprotic acid solution; the pH is largely dictated by the first ionization step because Ka1 is significantly larger than Ka2 and Ka3.
Solving Problems Involving Triprotic Acids
Solving problems involving triprotic acids often requires understanding equilibrium calculations and the use of ICE tables (Initial, Change, Equilibrium). These problems typically involve determining pH, concentrations of various species, or the amount of base needed for neutralization. These calculations can be complex, particularly when dealing with intermediate ionization steps, making the approximations used in simpler monoprotic calculations unreliable in triprotic acid scenarios.
Conclusion: Mastering the Concept of Triprotic Acids
Understanding the concept of triprotic acids, including their definition, characteristics, examples, and applications, is essential for anyone studying chemistry or related fields. The ability to distinguish triprotic acids from other acid types, interpret titration curves, and perform equilibrium calculations is crucial for successful problem-solving in this area. This knowledge will not only improve your chemical comprehension but will also help you appreciate the significant role these compounds play in various industries and natural processes. The stepwise ionization and the differences in the Ka values are key features that should be fully understood to grasp the nuanced chemical behavior of triprotic acids. This detailed exploration hopefully provides a robust understanding of this important class of chemical compounds.
Latest Posts
Latest Posts
-
Similarities Between A Square And A Rhombus
Mar 31, 2025
-
Coordination Of Balance And Body Movement Is Controlled By The
Mar 31, 2025
-
Why Are The Cells Generally Of A Small Size
Mar 31, 2025
-
Is Calcium Hydroxide A Base Or Acid
Mar 31, 2025
-
The Ultimate Source Of Energy From Fossil Fuels Is The
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Triprotic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
