Which Of The Following Is A Nonmetal
Juapaving
Mar 30, 2025 · 6 min read
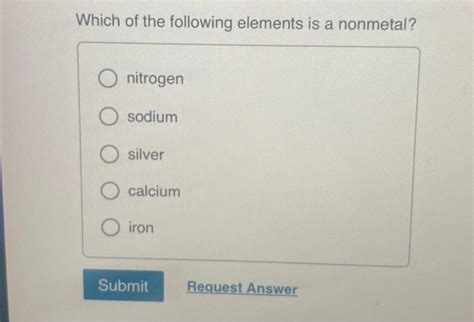
Table of Contents
Which of the Following is a Nonmetal? Understanding the Periodic Table and Chemical Properties
The question, "Which of the following is a nonmetal?" is a fundamental one in chemistry. Understanding the properties that define metals and nonmetals is crucial for grasping basic chemical principles and predicting how elements will behave. This comprehensive guide will delve deep into the world of nonmetals, explaining their characteristics, differentiating them from metals, and providing examples to solidify your understanding. We'll also explore the practical applications of nonmetals and their importance in our daily lives.
What are Metals and Nonmetals?
The periodic table is a powerful tool that organizes elements based on their atomic structure and properties. A key distinction within the table is the division between metals and nonmetals. This division isn't absolute; a band of elements called metalloids or semimetals exhibit properties of both metals and nonmetals.
Metals, located primarily on the left side of the periodic table, are generally characterized by:
- High electrical conductivity: They readily conduct electricity.
- High thermal conductivity: They efficiently transfer heat.
- Malleability: They can be hammered into thin sheets.
- Ductility: They can be drawn into wires.
- Metallic luster: They have a shiny appearance.
- High density: They are generally dense.
- High melting and boiling points: They require significant energy to change their state.
Nonmetals, mostly located on the right side of the periodic table, contrast sharply with metals, exhibiting:
- Poor electrical conductivity: They are generally poor conductors of electricity.
- Poor thermal conductivity: They are poor conductors of heat.
- Brittleness: They tend to be brittle and shatter when struck.
- Lack of metallic luster: They generally lack the shiny appearance of metals.
- Low density: They are generally less dense than metals.
- Low melting and boiling points: They often have relatively low melting and boiling points compared to metals.
Identifying Nonmetals: Key Characteristics and Examples
Several characteristics help identify nonmetals. Let's explore these characteristics further and then examine specific examples.
-
Formation of anions: Nonmetals tend to gain electrons to form negatively charged ions (anions) when they react with metals. This is due to their higher electronegativity, meaning they have a strong attraction for electrons.
-
Covalent bonding: Nonmetals predominantly form covalent bonds, where atoms share electrons rather than transferring them completely as in ionic bonds (characteristic of metal-nonmetal interactions). This results in molecules, rather than the crystal structures seen in many metals.
-
Gaseous or brittle solid state: At room temperature, many nonmetals exist as gases (like oxygen and nitrogen) or brittle solids (like sulfur and phosphorus).
-
Variety of colors and appearances: Unlike the usually silvery-grey appearance of most metals, nonmetals display a wide range of colors and appearances. For example, chlorine is a yellowish-green gas, while sulfur is a yellow solid.
-
Poor conductors of electricity and heat: This is a significant defining characteristic, separating them from highly conductive metals.
Examples of Nonmetals:
The following are some of the most common nonmetals, illustrating the diversity found within this group:
-
Hydrogen (H): The lightest element, usually considered a nonmetal despite its placement on the periodic table. It exists as a diatomic gas (H2).
-
Carbon (C): A fundamental element of life, forming the backbone of organic molecules. It exists in various allotropes (different forms of the same element), such as diamond (hardest natural substance) and graphite (used in pencils).
-
Nitrogen (N): A major component of the Earth's atmosphere (about 78%), existing as a diatomic gas (N2).
-
Oxygen (O): Essential for respiration, also a diatomic gas (O2) and a crucial part of many compounds.
-
Fluorine (F): The most reactive nonmetal, a pale yellow gas.
-
Chlorine (Cl): A yellowish-green gas used in water purification and other industrial processes.
-
Bromine (Br): The only nonmetal that is liquid at room temperature, a reddish-brown liquid.
-
Iodine (I): A dark grey, crystalline solid that sublimes (transitions directly from solid to gas).
-
Phosphorus (P): Exists in several allotropic forms, including white phosphorus (highly reactive and dangerous) and red phosphorus (less reactive).
-
Sulfur (S): A yellow, brittle solid, used in various industrial applications.
Metalloids: Bridging the Gap Between Metals and Nonmetals
Metalloids, or semimetals, occupy a region on the periodic table between metals and nonmetals. They exhibit properties intermediate between metals and nonmetals, making their classification less straightforward.
Examples of metalloids include:
- Boron (B)
- Silicon (Si)
- Germanium (Ge)
- Arsenic (As)
- Antimony (Sb)
- Tellurium (Te)
Metalloids' intermediate properties often lead to their use in semiconductor technology, where their ability to conduct electricity under specific conditions is crucial.
Applications of Nonmetals: From Everyday Life to Advanced Technology
Nonmetals are far from inert; they are essential components of many materials and processes crucial to our modern world.
-
Oxygen (O2): Essential for respiration in all aerobic organisms and vital for combustion processes.
-
Nitrogen (N2): Used in fertilizers to enhance agricultural productivity. It also serves as an inert gas in various industrial applications.
-
Carbon (C): Forms the basis of organic chemistry and is fundamental to life. Diamond is used in cutting tools, while graphite is used in pencils and batteries. Fullerenes and nanotubes, forms of carbon, hold tremendous potential in nanotechnology.
-
Chlorine (Cl2): Used in water purification and as a disinfectant. It also has applications in the production of various chemicals.
-
Fluorine (F): Used in the production of Teflon and other fluorocarbons, known for their non-stick properties. It also plays a role in dental hygiene products (fluoride).
-
Sulfur (S): Used in the production of sulfuric acid, a crucial industrial chemical used in many processes. It's also used in the vulcanization of rubber.
-
Phosphorus (P): Essential for life, playing a key role in DNA and energy transfer processes. It's also used in fertilizers and detergents.
Distinguishing Metals from Nonmetals: A Practical Approach
When asked "Which of the following is a nonmetal?", you should consider the following properties:
-
Location on the Periodic Table: Elements on the right side of the periodic table are more likely to be nonmetals.
-
Physical Properties: Check for characteristics like poor electrical and thermal conductivity, brittleness, lack of luster, and low melting/boiling points.
-
Chemical Properties: Consider their tendency to form covalent bonds and gain electrons to form anions.
By carefully analyzing these factors, you can effectively distinguish metals from nonmetals and confidently answer questions about the chemical classification of elements.
Conclusion: The Significance of Understanding Nonmetals
Understanding the distinction between metals and nonmetals, and the unique characteristics of nonmetals, is fundamental to a strong grasp of chemistry. Their diverse properties and widespread applications make them integral to various aspects of our lives, from the air we breathe to the technologies we rely upon. By mastering the concepts discussed in this article, you'll be well-equipped to navigate the world of chemical elements and their interactions. Remember, the periodic table is your friend – use it to guide your understanding and to confidently answer questions about the classification of elements. Continued learning and exploration of chemistry will further solidify your knowledge and appreciation for this fascinating field.
Latest Posts
Latest Posts
-
How Much Sides Does A Octagon Have
Apr 01, 2025
-
The Galapagos Finch Species Are An Excellent Example Of
Apr 01, 2025
-
Is The Nucleolus A Plant Or Animal Cell
Apr 01, 2025
-
What Is True About Irrational Numbers
Apr 01, 2025
-
What Is The Lcm Of 4 5 And 8
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
