Which Gas Is Not Greenhouse Gas
Juapaving
Mar 21, 2025 · 6 min read
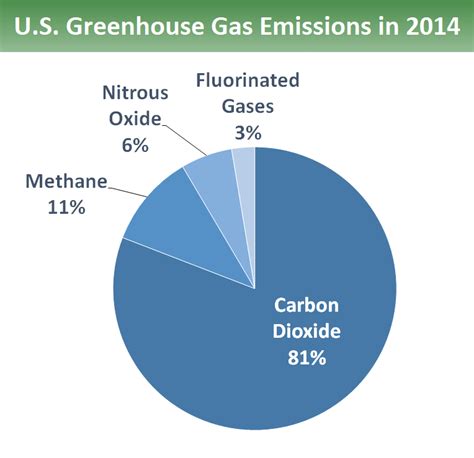
Table of Contents
Which Gas is Not a Greenhouse Gas? Understanding Atmospheric Composition and Climate Change
Climate change, driven largely by the enhanced greenhouse effect, is one of the most pressing challenges facing humanity. Understanding which gases contribute to this effect, and conversely, which do not, is crucial for developing effective mitigation strategies. While many gases trap heat in the atmosphere, some play a negligible or even a cooling role. This article delves into the atmospheric composition, exploring the gases that are not considered greenhouse gases, and examining the reasons behind their lack of radiative forcing.
The Greenhouse Effect: A Recap
Before identifying non-greenhouse gases, let's briefly revisit the greenhouse effect. Certain atmospheric gases absorb and re-emit infrared radiation (heat) emitted by the Earth's surface. This process traps heat within the atmosphere, making the planet warmer than it would be otherwise. This natural phenomenon is essential for life on Earth, maintaining a habitable temperature range. However, human activities have significantly increased the concentration of some greenhouse gases, leading to an enhanced greenhouse effect and global warming.
Key greenhouse gases include:
- Carbon Dioxide (CO2): The primary anthropogenic greenhouse gas, largely from fossil fuel combustion and deforestation.
- Methane (CH4): A potent greenhouse gas with a shorter atmospheric lifetime than CO2, emitted from agriculture, landfills, and natural gas leaks.
- Nitrous Oxide (N2O): Released from agricultural activities, industrial processes, and the burning of fossil fuels.
- Fluorinated Gases: Synthetic gases used in various industrial applications, possessing extremely high global warming potentials. Examples include hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6).
- Water Vapor (H2O): Although a potent greenhouse gas, its concentration is largely determined by temperature, creating a feedback loop rather than being a primary driver of climate change.
Gases That Are Not Greenhouse Gases
The majority of gases in the Earth's atmosphere are not significant greenhouse gases. Their molecular structure prevents them from effectively absorbing and re-emitting infrared radiation. This is primarily due to the lack of specific vibrational modes that resonate with the wavelengths of infrared radiation emitted by the Earth.
The most abundant gases in the atmosphere that don't contribute significantly to the greenhouse effect are:
1. Nitrogen (N2)
Nitrogen constitutes approximately 78% of the Earth's atmosphere. Its diatomic molecule (N2) is highly symmetrical and possesses a strong triple bond. This symmetrical structure means that its vibrational modes do not effectively interact with infrared radiation. Consequently, nitrogen plays a negligible role in the greenhouse effect.
Key Characteristics:
- Abundance: ~78% of the atmosphere
- Molecular Structure: Diatomic (N2) with a strong triple bond and symmetrical structure.
- Infrared Absorption: Negligible.
2. Oxygen (O2)
Oxygen makes up roughly 21% of the Earth's atmosphere and is crucial for respiration and many other biological processes. Similar to nitrogen, the symmetrical diatomic molecule (O2) and its vibrational modes do not effectively interact with infrared radiation. Therefore, it is not a significant greenhouse gas.
Key Characteristics:
- Abundance: ~21% of the atmosphere
- Molecular Structure: Diatomic (O2) with a symmetrical structure.
- Infrared Absorption: Negligible.
3. Argon (Ar)
Argon is a noble gas, constituting about 0.93% of the Earth's atmosphere. Noble gases are monatomic, meaning they exist as single atoms. They do not possess any vibrational modes capable of interacting with infrared radiation, thus they are not greenhouse gases.
Key Characteristics:
- Abundance: ~0.93% of the atmosphere
- Molecular Structure: Monatomic.
- Infrared Absorption: Negligible.
4. Neon (Ne), Helium (He), Krypton (Kr), Xenon (Xe)
These other noble gases are present in the atmosphere in trace amounts. Like argon, their monatomic structure means they lack the necessary vibrational modes to absorb infrared radiation, making them insignificant to the greenhouse effect.
Key Characteristics:
- Abundance: Trace amounts.
- Molecular Structure: Monatomic.
- Infrared Absorption: Negligible.
The Importance of Understanding Non-Greenhouse Gases
While these gases do not contribute to the greenhouse effect, their presence in the atmosphere is critical for various reasons. Nitrogen and oxygen are fundamental to life on Earth. Argon and other noble gases have industrial applications. Understanding the role of each atmospheric component, including the non-greenhouse gases, is essential for comprehensive atmospheric modeling and climate change research. Accurate models of atmospheric dynamics must account for all constituent gases and their interactions.
Distinguishing Between Greenhouse and Non-Greenhouse Gases: A Deeper Dive
The key distinction between greenhouse and non-greenhouse gases lies in their molecular structure and the way they interact with infrared radiation. Greenhouse gases possess asymmetrical molecular structures with vibrational modes that can absorb and re-emit infrared radiation at specific wavelengths. This absorption and re-emission process traps heat within the atmosphere.
In contrast, gases like nitrogen, oxygen, and the noble gases have symmetrical molecular structures. Their vibrational modes are not effectively coupled to infrared radiation, preventing them from absorbing and re-emitting heat. Therefore, they do not contribute significantly to the greenhouse effect.
The Role of Molecular Symmetry and Vibrational Modes
The symmetry of a molecule plays a critical role in its ability to interact with infrared radiation. Molecules with a center of symmetry, like N2 and O2, generally do not absorb infrared radiation. This is because the changes in dipole moment associated with their vibrational modes are zero. The dipole moment is a measure of the separation of positive and negative charges within a molecule. A change in dipole moment is required for a molecule to interact with infrared radiation.
Asymmetrical molecules, such as CO2, CH4, and H2O, lack a center of symmetry and exhibit changes in dipole moment during their vibrational modes. These changes allow them to absorb and re-emit infrared radiation, contributing to the greenhouse effect.
Beyond the Basics: Factors Influencing Greenhouse Gas Effects
While molecular structure is the primary determinant of a gas's greenhouse effect, other factors also play a role. These include:
-
Atmospheric Lifetime: Some greenhouse gases, like methane, have shorter atmospheric lifetimes than others, like carbon dioxide. While methane is a more potent greenhouse gas on a per-molecule basis, its shorter lifetime means its overall impact over time may be less than CO2.
-
Global Warming Potential (GWP): GWP is a measure of how much heat a greenhouse gas traps in the atmosphere compared to carbon dioxide over a specific time period (usually 100 years). Different gases have different GWPs.
-
Concentration: The concentration of a gas in the atmosphere significantly impacts its contribution to the greenhouse effect. Even gases with high GWPs have a relatively small impact if their atmospheric concentration is very low.
Conclusion: A Holistic View of Atmospheric Composition
Understanding which gases are and are not greenhouse gases is crucial for comprehending climate change and developing effective mitigation strategies. While the focus is often on reducing greenhouse gas emissions, recognizing the role of non-greenhouse gases in the overall atmospheric composition is equally important for accurate climate modeling and informed decision-making. The interplay between these gases, their concentrations, and their radiative properties shapes the Earth's climate system and ultimately determines the planet's habitability. Further research and monitoring of atmospheric composition are essential to improve our understanding and response to the challenges of climate change. A comprehensive approach that considers both greenhouse and non-greenhouse gases is crucial for developing effective strategies for a sustainable future.
Latest Posts
Latest Posts
-
How Many Liters Are In 7 Gallons
Mar 27, 2025
-
Why Does An Equation Need To Be Balanced
Mar 27, 2025
-
What Does The Slope Of A Distance Time Graph Indicate
Mar 27, 2025
-
What Word Describes The Equal Shares Of A Shape
Mar 27, 2025
-
A Nucleotide Consists Of Three Parts
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Which Gas Is Not Greenhouse Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
