Which Functional Group Is Found In Methanol
Juapaving
Mar 30, 2025 · 6 min read
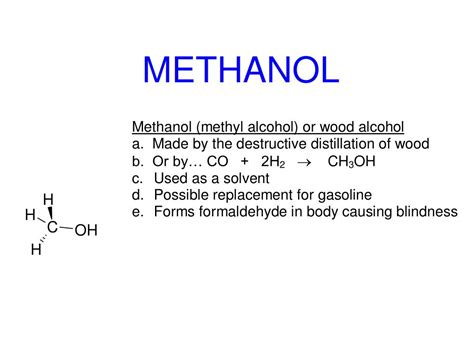
Table of Contents
Which Functional Group is Found in Methanol? A Deep Dive into Alcohols
Methanol, the simplest alcohol, holds a pivotal position in organic chemistry. Understanding its structure and, more specifically, its functional group is crucial for grasping its properties and reactivity. This comprehensive article will delve into the functional group present in methanol, exploring its characteristics, nomenclature, and its impact on the molecule's behavior. We'll also examine related concepts, ensuring a thorough understanding of this fundamental organic compound.
Understanding Functional Groups: The Building Blocks of Organic Chemistry
Before diving into methanol's specific functional group, let's establish a foundational understanding of what functional groups are. In organic chemistry, a functional group is a specific atom or group of atoms within a molecule that is responsible for the characteristic chemical reactions of that molecule. These groups dictate how a molecule will behave in various chemical environments, influencing its reactivity, solubility, and other properties. They are essentially the reactive centers of an organic molecule. Different functional groups confer different properties. For example, the presence of a hydroxyl group (-OH) drastically changes the properties of a hydrocarbon, while a carboxyl group (-COOH) introduces acidic behavior.
Identifying the Functional Group in Methanol (CH₃OH)
Methanol, with its chemical formula CH₃OH, is a simple organic molecule. It contains a hydroxyl group (-OH), which is bonded to a methyl group (CH₃). This hydroxyl group is the key functional group in methanol, and it's responsible for methanol's characteristic properties as an alcohol. The presence of this –OH group is what distinguishes methanol from methane (CH₄), a simple hydrocarbon.
The Hydroxyl Group (-OH): A Detailed Examination
The hydroxyl group consists of an oxygen atom covalently bonded to a hydrogen atom. This bond is highly polar due to the significant difference in electronegativity between oxygen and hydrogen. Oxygen is far more electronegative than hydrogen, meaning it attracts the shared electrons more strongly. This leads to a partial negative charge (δ-) on the oxygen atom and a partial positive charge (δ+) on the hydrogen atom. This polarity is the cornerstone of many of the hydroxyl group's characteristic reactions and properties.
Methanol's Structure and the Hydroxyl Group's Influence
The hydroxyl group's presence dramatically impacts methanol's properties. Here's how:
-
Polarity: The polar hydroxyl group makes methanol a polar molecule. This polarity allows methanol to form hydrogen bonds with water molecules, making it highly soluble in water. This contrasts sharply with methane, which is nonpolar and largely insoluble in water.
-
Hydrogen Bonding: The ability of methanol to form hydrogen bonds is a direct consequence of the hydroxyl group. The hydrogen atom of the hydroxyl group can form a hydrogen bond with a lone pair of electrons on the oxygen atom of another methanol molecule or a water molecule. These hydrogen bonds significantly influence methanol's boiling point, which is much higher than that of methane, despite their similar molecular weights.
-
Acidity: While not a strong acid, methanol exhibits weak acidic properties due to the hydroxyl group. The oxygen atom can donate a proton (H⁺) under specific conditions, although this is less readily done compared to carboxylic acids or mineral acids. The resulting methoxide ion (CH₃O⁻) is a relatively strong base.
-
Reactivity: The hydroxyl group is a reactive site within the methanol molecule. It can participate in various reactions, such as esterification (reaction with carboxylic acids to form esters) and dehydration (loss of water molecule to form ethers).
Nomenclature and Classification of Alcohols
Methanol belongs to the broader class of organic compounds called alcohols. Alcohols are organic compounds characterized by the presence of a hydroxyl (-OH) group attached to a saturated carbon atom (a carbon atom bonded to four other atoms via single bonds). The nomenclature of alcohols follows specific rules. The name of the parent alkane (the longest carbon chain) is modified by replacing the "-e" ending with "-ol." For methanol, the parent alkane is methane (one carbon atom), so the name becomes methanol.
Different Types of Alcohols
Alcohols can be further classified based on the number of alkyl groups attached to the carbon atom bearing the hydroxyl group:
-
Primary (1°) alcohols: The carbon atom bearing the hydroxyl group is bonded to only one other carbon atom (e.g., methanol, ethanol).
-
Secondary (2°) alcohols: The carbon atom bearing the hydroxyl group is bonded to two other carbon atoms.
-
Tertiary (3°) alcohols: The carbon atom bearing the hydroxyl group is bonded to three other carbon atoms.
Methanol is a primary alcohol, as the carbon atom attached to the hydroxyl group is bonded only to one other carbon atom (a hydrogen atom in this case).
Applications of Methanol
Methanol's unique properties, stemming from its hydroxyl functional group, have led to numerous applications across various industries. Some key applications include:
-
Fuel: Methanol is used as a fuel, both directly and as an additive in gasoline. Its combustion produces carbon dioxide and water, making it a relatively cleaner-burning fuel compared to gasoline.
-
Solvent: Methanol is a versatile solvent used in various industrial processes, including the production of paints, varnishes, and resins. Its polarity and ability to dissolve both polar and nonpolar compounds make it an ideal solvent for many applications.
-
Chemical Intermediate: Methanol serves as a crucial building block for the synthesis of other important chemicals, including formaldehyde, acetic acid, and methyl tert-butyl ether (MTBE), a gasoline additive.
-
Antifreeze: Methanol's low freezing point makes it useful as an antifreeze in certain applications.
-
Fuel cells: Methanol is used as fuel in direct methanol fuel cells (DMFCs) which directly convert chemical energy to electrical energy.
Safety Considerations of Methanol
While methanol has numerous applications, it is crucial to recognize its toxicity. Methanol is highly toxic and can cause blindness or even death if ingested. It is metabolized in the liver to formaldehyde and formic acid, both of which are highly toxic substances. Therefore, appropriate safety precautions must always be taken when handling methanol.
Conclusion: The Hydroxyl Group's Crucial Role
The hydroxyl functional group is the defining characteristic of methanol, dictating its polarity, hydrogen bonding capability, acidity, and reactivity. This simple yet significant group is responsible for methanol's solubility in water, its higher boiling point compared to similar hydrocarbons, and its ability to participate in various chemical reactions. Understanding the hydroxyl group's influence is critical to comprehending methanol's properties, its wide range of applications, and its associated safety concerns. This detailed exploration demonstrates the importance of functional groups in determining the behavior and usefulness of organic molecules. The hydroxyl group in methanol, specifically, showcases how a small structural feature can have a profound impact on the properties and applications of a chemical compound. Further study into other functional groups and their influence on different organic molecules will further solidify this foundational understanding.
Latest Posts
Latest Posts
-
Mario Has To Take Medication For 180 Days
Apr 01, 2025
-
What Is On A Physical Map
Apr 01, 2025
-
P Block Elements In Periodic Table
Apr 01, 2025
-
Adjective That Starts With L To Describe A Mom
Apr 01, 2025
-
Is 35 A Multiple Of 6
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Functional Group Is Found In Methanol . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
