Which Element Below Is Least Reactive
Juapaving
Mar 11, 2025 · 5 min read
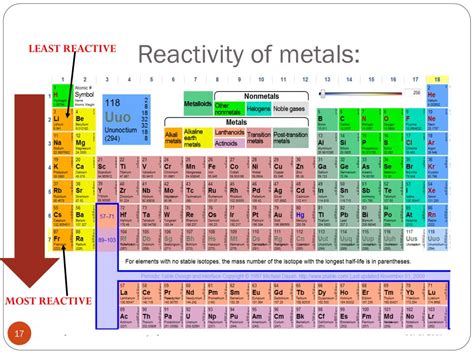
Table of Contents
Which Element Below is Least Reactive? Understanding Chemical Reactivity
The question "which element below is least reactive?" hinges on understanding chemical reactivity itself. Reactivity isn't a simple yes/no characteristic; it's a complex interplay of factors determined by an element's electronic structure and its position on the periodic table. This article delves deep into the concept of reactivity, examining the factors that influence it and providing a framework for determining which elements exhibit the least tendency to react.
Understanding Chemical Reactivity
Chemical reactivity describes an element's tendency to undergo chemical changes, meaning the formation or breaking of chemical bonds. Highly reactive elements readily participate in reactions, often vigorously, while less reactive elements require specific conditions or react much more slowly. This reactivity is fundamentally linked to an element's electron configuration, specifically its valence electrons.
Valence Electrons: The Key Players
Valence electrons are the outermost electrons in an atom. They are the electrons most involved in chemical bonding, as they are the ones that interact with other atoms. Elements strive for stability, often achieving this by filling their outermost electron shell. This desire for stability drives their reactivity.
-
Noble Gases (Group 18): The noble gases (Helium, Neon, Argon, Krypton, Xenon, Radon) possess a full valence shell. This stable octet (or duet for Helium) makes them incredibly unreactive, explaining why they are often called "inert gases." They rarely form compounds.
-
Halogens (Group 17): Halogens (Fluorine, Chlorine, Bromine, Iodine, Astatine) have seven valence electrons. They are highly reactive because they readily gain one electron to achieve a stable octet, forming negative ions.
-
Alkali Metals (Group 1): Alkali metals (Lithium, Sodium, Potassium, Rubidium, Cesium, Francium) have only one valence electron. They readily lose this electron to form positive ions, achieving a stable electron configuration. Their reactivity increases as you go down the group.
-
Alkaline Earth Metals (Group 2): Alkaline earth metals (Beryllium, Magnesium, Calcium, Strontium, Barium, Radium) have two valence electrons. They tend to lose these two electrons to form positive ions, but their reactivity is lower than that of alkali metals.
-
Transition Metals: Transition metals exhibit a more complex reactivity pattern, often forming multiple oxidation states. Their reactivity is influenced by factors beyond just their valence electrons.
Factors Influencing Reactivity
Several factors beyond the number of valence electrons influence an element's reactivity:
1. Ionization Energy:**
Ionization energy is the energy required to remove an electron from a neutral atom. Elements with low ionization energies readily lose electrons and are thus more reactive. Alkali metals, for instance, have low ionization energies.
2. Electronegativity:
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Highly electronegative elements tend to attract electrons strongly, making them more likely to gain electrons and form negative ions. Halogens are highly electronegative.
3. Atomic Radius:
Atomic radius refers to the size of an atom. As you move down a group in the periodic table, atomic radius increases. This increase in size makes it easier to remove valence electrons, resulting in increased reactivity for alkali metals and alkaline earth metals as you go down the group.
4. Shielding Effect:
The inner electrons shield the outer valence electrons from the positive charge of the nucleus. The stronger the shielding effect, the weaker the attraction between the nucleus and valence electrons, making it easier to remove valence electrons and increasing reactivity.
5. Electron Affinity:
Electron affinity is the energy change that occurs when an atom gains an electron. A high electron affinity indicates a strong tendency to gain electrons, enhancing reactivity.
Identifying the Least Reactive Element: A Comparative Analysis
Given the factors discussed above, the least reactive elements are undoubtedly the noble gases. Their full valence shells provide exceptional stability, making them extremely reluctant to participate in chemical reactions under normal conditions. While some heavier noble gases can be forced to react under extreme conditions (high pressure, high energy), their inherent unreactivity is undeniable.
Let's compare the reactivity of different groups:
-
Noble Gases vs. Halogens: Noble gases are significantly less reactive than halogens. Halogens readily gain one electron, while noble gases have no such driving force.
-
Noble Gases vs. Alkali Metals: Alkali metals readily lose one electron, while noble gases have no tendency to lose or gain electrons. Noble gases are far less reactive.
-
Noble Gases vs. Transition Metals: Transition metals exhibit variable reactivity, but none approach the inertness of noble gases.
Specific Examples and Context
The question "which element below is least reactive" requires a list of elements for a definitive answer. However, without that list, we can confidently state that if a noble gas (Helium, Neon, Argon, Krypton, Xenon, Radon) is among the options, it will be the least reactive.
If the list contains only metals, the least reactive metal would likely be one of the transition metals, possibly one found towards the right of the transition block. Their relatively higher ionization energies and complex electronic configurations contribute to lower reactivity compared to the alkali or alkaline earth metals.
Conclusion: The Inertness of Noble Gases
The least reactive elements are consistently the noble gases due to their complete valence electron shells. This stability makes them exceptional examples of minimal chemical reactivity under ordinary conditions. Understanding the factors influencing reactivity, including ionization energy, electronegativity, atomic radius, shielding effect, and electron affinity, provides a comprehensive framework for assessing an element's propensity to undergo chemical change. Therefore, when presented with a multiple-choice question asking for the least reactive element, look for a noble gas in the options. If a noble gas is not present, a thorough evaluation of the other provided elements based on the factors discussed above is necessary.
Latest Posts
Latest Posts
-
Examples Of Monocot Plants And Dicot Plants
Mar 12, 2025
-
Land Surrounded By Three Sides Of Water
Mar 12, 2025
-
What Is Lii In Roman Numerals
Mar 12, 2025
-
Is Cooking An Egg Endothermic Or Exothermic
Mar 12, 2025
-
What Is The Unit Of Measuring Temperature
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Which Element Below Is Least Reactive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
