What Is The Unit Of Measuring Temperature
Juapaving
Mar 12, 2025 · 6 min read
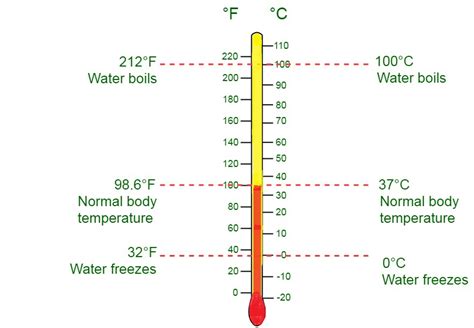
Table of Contents
What is the Unit of Measuring Temperature? A Deep Dive into Thermometry
Temperature, a fundamental concept in physics and everyday life, measures the average kinetic energy of particles within a system. Understanding temperature requires grasping not only its meaning but also the various units used to quantify it. This comprehensive guide delves into the intricacies of temperature measurement, exploring the history, science, and practical applications of different temperature units.
The Importance of Temperature Measurement
Accurate temperature measurement is crucial across numerous disciplines. From weather forecasting and climate science to medical diagnostics and industrial processes, precise temperature readings are essential for effective monitoring, control, and analysis. Variations in temperature can significantly impact chemical reactions, biological processes, and the physical properties of materials. In everyday life, we rely on temperature readings to regulate our comfort, cook food safely, and maintain the proper functioning of various appliances.
A Brief History of Thermometry
The quest to accurately measure temperature has a rich history. Early attempts relied on subjective observations, such as the feeling of "hot" or "cold." However, the development of reliable thermometers revolutionized temperature measurement.
Early Thermometers: Qualitative to Quantitative
Early thermometers, often based on the expansion of liquids like alcohol or mercury, lacked standardization. Different makers used different scales, resulting in inconsistent readings. The invention of the first reliable thermometer is often attributed to Galileo Galilei in the late 16th century, although his "thermoscope" lacked a standardized scale.
The Emergence of Standardized Scales: Celsius and Fahrenheit
The 18th century witnessed the development of standardized temperature scales, primarily the Celsius (formerly Centigrade) and Fahrenheit scales. Anders Celsius, a Swedish astronomer, proposed a scale in 1742 that used the freezing and boiling points of water as reference points, defining 0°C and 100°C respectively. Gabriel Fahrenheit, a German physicist, developed his own scale around the same time, setting 32°F as the freezing point of water and 212°F as its boiling point.
Common Units of Temperature Measurement
Several units are commonly used to measure temperature, each with its own advantages and disadvantages. The most prevalent include:
1. Celsius (°C)
The Celsius scale, widely used globally, defines 0°C as the freezing point of water and 100°C as its boiling point at standard atmospheric pressure. Its simplicity and widespread adoption make it the preferred scale for many scientific and everyday applications. The Celsius scale is part of the International System of Units (SI), although the SI unit for temperature is actually the Kelvin.
Advantages:
- Widely used and understood: Its familiarity simplifies communication and data interpretation.
- Intuitive scale: The freezing and boiling points of water provide readily understandable reference points.
- Suitable for most everyday applications: Its range covers the temperatures typically experienced in daily life.
Disadvantages:
- Not absolute: Celsius uses arbitrary reference points, unlike the Kelvin scale.
2. Fahrenheit (°F)
The Fahrenheit scale is primarily used in the United States and a few other countries. It sets the freezing point of water at 32°F and the boiling point at 212°F. Its origins are rooted in early thermometry, and while less prevalent internationally, its continued use in certain regions necessitates understanding its conversion to other scales.
Advantages:
- Historically established: Its long-standing presence in some regions means its continued use is entrenched.
Disadvantages:
- Less intuitive than Celsius: Its arbitrary reference points make it less easily grasped than the Celsius scale.
- Limited international usage: Its restricted use hampers global data sharing and collaboration.
- More complex conversions: Converting between Fahrenheit and other scales requires more complex mathematical operations compared to Celsius.
3. Kelvin (K)
The Kelvin scale, the base unit of thermodynamic temperature in the International System of Units (SI), provides an absolute temperature scale. It defines absolute zero, the theoretical point where all molecular motion ceases, as 0 K. The size of a Kelvin degree is the same as a Celsius degree, meaning a change of 1 K is equivalent to a change of 1°C.
Advantages:
- Absolute scale: Its zero point represents the absence of thermal energy, providing a fundamental reference.
- Essential for scientific calculations: Many scientific formulas and calculations require using the Kelvin scale.
- Provides a consistent basis for thermodynamics: The Kelvin scale forms the foundation of thermodynamic principles.
Disadvantages:
- Less intuitive for everyday use: The concept of absolute zero is less relatable than the freezing and boiling points of water.
4. Rankine (°R)
The Rankine scale is an absolute temperature scale that uses Fahrenheit degrees. Absolute zero is defined as 0°R, and the size of a Rankine degree is the same as a Fahrenheit degree. It's less common than Kelvin or Celsius and primarily used in some engineering applications.
Advantages:
- Consistent with Fahrenheit: Useful when working with Fahrenheit-based systems.
Disadvantages:
- Limited usage: It's not widely used compared to Kelvin or Celsius.
Temperature Conversions: Bridging the Scales
Converting between different temperature scales is often necessary. The following formulas facilitate these conversions:
- Celsius to Fahrenheit: °F = (°C × 9/5) + 32
- Fahrenheit to Celsius: °C = (°F - 32) × 5/9
- Celsius to Kelvin: K = °C + 273.15
- Kelvin to Celsius: °C = K - 273.15
- Fahrenheit to Rankine: °R = °F + 459.67
- Rankine to Fahrenheit: °F = °R - 459.67
These formulas are crucial for ensuring consistent and accurate temperature readings across various applications. Many online calculators and conversion tools can simplify this process.
Temperature Measurement Devices: Tools of the Trade
Various instruments measure temperature, each suited to specific applications:
-
Thermometers: These are common devices using the expansion of liquids (mercury or alcohol) or the change in electrical resistance (thermistors) to measure temperature. Digital thermometers offer greater precision and convenience.
-
Thermocouples: These devices use the Seebeck effect, producing a voltage proportional to the temperature difference between two dissimilar metals. They are widely used in industrial settings due to their robustness and wide temperature range.
-
Pyrometers: These non-contact thermometers measure the thermal radiation emitted by an object, allowing for temperature measurement from a distance. They are invaluable for measuring high temperatures in inaccessible locations.
-
Infrared thermometers: Similar to pyrometers, infrared thermometers measure temperature using infrared radiation. They are commonly used for quick, non-contact temperature checks in various applications.
Applications Across Diverse Fields
The applications of accurate temperature measurement extend far and wide:
1. Meteorology and Climate Science
Temperature plays a crucial role in weather forecasting and climate change research. Accurate temperature data helps predict weather patterns, monitor climate trends, and assess the impact of global warming.
2. Medicine and Healthcare
Precise temperature measurements are essential in medical diagnostics and treatment. Body temperature is a critical indicator of health, and temperature monitoring plays a vital role in patient care and disease management.
3. Food Industry
Temperature control is crucial in food processing, storage, and preservation. Accurate temperature measurement ensures food safety, prevents spoilage, and maintains the quality of food products.
4. Manufacturing and Industry
Industrial processes rely on precise temperature control for efficiency and quality. Temperature monitoring is essential in various manufacturing processes, including chemical reactions, metalworking, and electronics production.
5. Scientific Research
Accurate temperature measurement underpins numerous scientific experiments and research. From studying the properties of materials to conducting biological experiments, precise temperature control is crucial for reliable results.
Conclusion: Understanding the Language of Heat
Temperature measurement is a fundamental aspect of scientific inquiry and technological advancement. The various units and tools used in thermometry provide the means to quantify heat, enabling us to understand and manipulate its effects across numerous domains. From the Celsius scale's global prevalence to the Kelvin scale's scientific significance, each unit contributes to our comprehensive understanding of temperature, its effects, and its critical role in our world. Choosing the appropriate unit depends largely on the context, application, and desired precision. Mastering the intricacies of temperature measurement is vital for anyone engaging in scientific research, industrial processes, or simply understanding the world around us.
Latest Posts
Latest Posts
-
Is 19 A Prime Number Or A Composite Number
May 09, 2025
-
Is Force Increase On An Inclined Plane
May 09, 2025
-
Interesting Words That Start With V
May 09, 2025
-
Compare And Contrast A Light Microscope And An Electron Microscope
May 09, 2025
-
What Is 6 25 Meters In Feet And Inches
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Unit Of Measuring Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.