How Many Valence Electrons Does Copper Have
Juapaving
Mar 10, 2025 · 5 min read
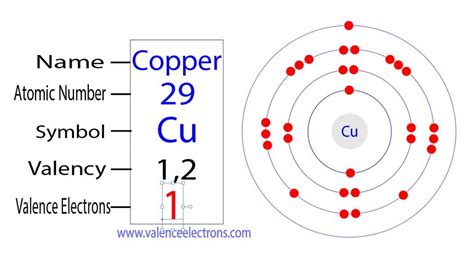
Table of Contents
How Many Valence Electrons Does Copper Have? A Deep Dive into Electronic Configuration and Chemical Behavior
Copper, a reddish-brown metal with excellent electrical conductivity, plays a crucial role in numerous applications, from electrical wiring to plumbing and even biological processes. Understanding its chemical behavior is key to appreciating its widespread use. A fundamental aspect of this understanding lies in determining the number of valence electrons it possesses. This article will delve deep into the electronic configuration of copper, explaining why determining its valence electrons isn't as straightforward as it might seem, and exploring the implications of its unique electronic structure on its chemical properties.
Understanding Valence Electrons
Before we tackle copper specifically, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the most loosely bound and, therefore, are the ones primarily involved in chemical bonding and determining an element's reactivity. The number of valence electrons dictates how an atom will interact with other atoms, forming ionic or covalent bonds.
The number of valence electrons can usually be easily determined from an element's position in the periodic table. For main group elements (groups 1-18, excluding transition metals), the group number directly corresponds to the number of valence electrons. However, this simple rule doesn't always apply to transition metals, including copper.
Copper's Electronic Configuration: The Unexpected Twist
Copper's atomic number is 29, meaning it has 29 electrons. Based on its position in the periodic table (Group 11), one might expect it to have one valence electron. However, the actual electronic configuration of copper presents a fascinating anomaly. Instead of the expected [Ar] 3d<sup>9</sup> 4s<sup>2</sup> configuration, copper exhibits the configuration [Ar] 3d<sup>10</sup> 4s<sup>1</sup>.
This seemingly small change has significant consequences. The 4s subshell is generally filled before the 3d subshell, but in the case of copper (and other transition metals like chromium), a single electron from the 4s subshell moves into the 3d subshell. This is primarily due to the increased stability associated with a completely filled 3d subshell. A completely filled or half-filled subshell provides extra stability due to electron-electron repulsion minimization and favorable exchange energy.
Why the Electron Configuration Matters
This seemingly minor shift has profound implications for copper's properties:
- Enhanced Stability: A completely filled 3d subshell results in increased stability, making copper less reactive than one might expect from a simple consideration of its group number.
- Variable Oxidation States: While copper commonly exhibits a +2 oxidation state (losing two electrons), it can also display a +1 oxidation state, thanks to the single 4s electron. This versatility in oxidation states is a hallmark of transition metals and accounts for copper's varied chemical behavior.
Determining Copper's Valence Electrons: A Case for Ambiguity
The unique electronic configuration of copper introduces a level of ambiguity in defining the number of valence electrons. While some might argue for one valence electron (the single 4s electron), others might include the 3d electrons, resulting in a higher count. In reality, the situation is more nuanced:
- One Valence Electron (Simple Model): This perspective considers only the outermost 4s electron as the valence electron. This model aligns with the common oxidation state (+1) of copper.
- Eleven Valence Electrons (Extended Model): This viewpoint takes into account both the 4s and 3d electrons as valence electrons. It better explains the possibility of the +2 oxidation state, where copper loses both the 4s and one 3d electron.
The Practical Implications
In practice, both viewpoints have merit, depending on the context. For simpler chemical reactions involving the +1 oxidation state, considering only one valence electron might suffice. However, for more complex reactions or those involving the +2 oxidation state, considering the participation of 3d electrons provides a more accurate description.
Copper's Chemical Behavior and Valence Electrons
The variable valence electron count is reflected in copper's varied chemical behavior. Let's explore some examples:
Copper(I) Compounds (+1 Oxidation State):
In compounds like copper(I) oxide (Cu<sub>2</sub>O) and copper(I) chloride (CuCl), copper exhibits a +1 oxidation state. This implies the involvement of only the 4s electron in bonding. These compounds are typically white or pale yellow, demonstrating a significant departure from copper's reddish-brown metallic appearance.
Copper(II) Compounds (+2 Oxidation State):
In compounds like copper(II) oxide (CuO) and copper(II) sulfate (CuSO<sub>4</sub>), copper adopts a +2 oxidation state. This indicates the participation of both the 4s and one 3d electron in forming bonds. These compounds often exhibit characteristic blue or green colors.
Copper's Role in Biology and its Valence Electrons
Copper's presence in biological systems further highlights its unique electronic structure. It plays crucial roles in various enzymes, acting as a cofactor. Its ability to exist in both +1 and +2 oxidation states allows it to participate in redox reactions, essential for numerous biological processes.
The copper-containing enzyme cytochrome c oxidase, a crucial component of the electron transport chain in cellular respiration, exemplifies this. The ability of copper to switch between oxidation states facilitates electron transfer, contributing to ATP production. The participation of both 4s and 3d electrons in the electron transfer process within these enzymes emphasizes the significance of considering both when evaluating copper's electronic behavior in biological contexts.
Conclusion: The Importance of Context in Defining Valence Electrons
Determining the precise number of valence electrons in copper is context-dependent. The simple model of one valence electron suffices for explaining its +1 oxidation state and some basic chemical reactions. However, for a complete understanding of its diverse chemical behavior and participation in more complex processes, such as the +2 oxidation state and biological functions, considering both 4s and 3d electrons is essential. Copper's unique electronic configuration highlights the intricate relationship between electronic structure and chemical properties, reminding us that simplification can sometimes overshadow the rich complexity of nature's chemistry. Further research continually expands our understanding of this versatile metal and its remarkable role in various fields. Understanding the nuances of its electronic structure is key to unlocking the full potential of its applications.
Latest Posts
Latest Posts
-
Whats The Difference Between Autotroph And Heterotroph
Mar 10, 2025
-
What Is 4 5 As A Decimal
Mar 10, 2025
-
Highest Common Factor Of 9 And 12
Mar 10, 2025
-
Where Is The Mass Of The Atom Located
Mar 10, 2025
-
What Energy Is Transfered In A Genorator
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Copper Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
