Where Is Hydrogen In The Periodic Table
Juapaving
Mar 23, 2025 · 5 min read
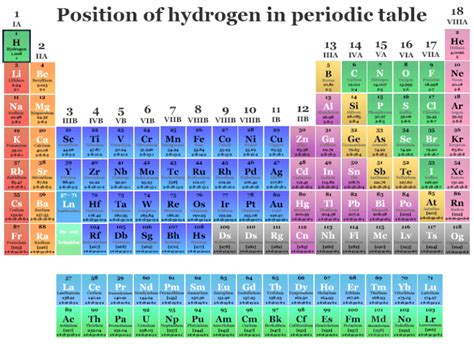
Table of Contents
Where is Hydrogen in the Periodic Table? Unpacking the Unique Properties of the First Element
Hydrogen, the simplest and most abundant element in the universe, holds a unique position in the periodic table. Its placement, however, isn't always straightforward, sparking debate and highlighting its exceptional characteristics. This comprehensive exploration delves into hydrogen's location, its peculiar properties, and why its position is a subject of ongoing scientific discussion.
Hydrogen's Placement: A Tale of Two Groups
You'll typically find hydrogen nestled in the top left corner of the periodic table, often placed above the alkali metals (Group 1). This placement reflects its electronic configuration, with a single electron in its outermost shell, similar to the alkali metals like lithium, sodium, and potassium. This single electron can be easily lost, leading to the formation of a +1 ion, H⁺. This is a key characteristic that shares similarity with alkali metals. This is why hydrogen is often considered an alkali metal.
However, this isn't the whole story. Hydrogen also exhibits properties that align it with the halogens (Group 17). This is because its electron shell can also gain an electron, forming a hydride ion (H⁻) with a -1 charge. This characteristic mirrors the behavior of halogens such as fluorine, chlorine, and bromine which gain an electron to achieve a stable electron configuration.
The Ambiguity of Hydrogen's Properties
This dual nature is what makes hydrogen's position so intriguing. It doesn't perfectly fit into either Group 1 or Group 17. It's not quite an alkali metal, nor is it a true halogen. Its behavior depends heavily on the context and the specific chemical reaction.
Its unique characteristics:
-
Electron Configuration: The simplest of all elements. Its solitary electron is easily shared or exchanged, leading to diverse chemical behaviours.
-
Oxidation States: Exhibits a wide range of oxidation states, including +1, -1, and 0, further demonstrating its versatility in chemical bonding.
-
Isotopes: Possesses three naturally occurring isotopes: protium (¹H), deuterium (²H or D), and tritium (³H or T), each varying in their neutron count. These isotopes show subtle differences in their properties, significantly impacting applications such as nuclear fusion and magnetic resonance imaging (MRI).
-
Bonding Capabilities: Forms covalent bonds with other nonmetals, sharing electrons to achieve stability. With metals, it can form ionic bonds through electron transfer, generating hydrides (e.g., LiH, NaH). Furthermore, it participates in metallic bonding in certain metal hydrides.
-
Physical States: Exists in three states at different temperatures and pressures. It is a gas under normal conditions, making it highly compressible and manageable in industrial settings. The solid form of hydrogen is of high scientific interest for its potential use in energy storage and superconductivity.
-
Abundance: Is the most abundant element in the universe but is relatively less abundant in Earth’s atmosphere. It is commonly found bonded with other elements such as oxygen in water and carbon in hydrocarbons.
Why Hydrogen Doesn't Perfectly Fit Into Any Group
Hydrogen's unique position is a direct consequence of its electronic structure and the way it interacts with other elements. Unlike other elements with multiple electron shells, hydrogen only has one shell, containing just one electron. This makes its bonding behaviour profoundly different. Alkali metals, although also having one electron in their outermost shell, possess inner electron shells that significantly influence their properties and chemical reactivity.
The Periodic Table's Evolution and Hydrogen's Placement
The periodic table itself is a dynamic representation of our understanding of chemical elements. The placement of hydrogen hasn't always been consistent. Early versions often placed hydrogen separately, recognizing its distinct nature. As our understanding of atomic structure and chemical bonding improved, the need to place it within a group became increasingly apparent, leading to its current, yet somewhat ambiguous, placement. Therefore, it can often be found in its own separate category atop the table.
Hydrogen's Role in Chemistry and Beyond
Hydrogen's unique properties have made it a cornerstone element across various fields:
Industrial Applications:
-
Ammonia Production (Haber-Bosch Process): Hydrogen is crucial for synthesizing ammonia, a vital component of fertilizers.
-
Petroleum Refining: Used in hydrocracking and hydrodesulfurization processes to improve the quality of petroleum products.
-
Metal Refining: Plays a role in refining various metals such as copper and iron.
-
Fuel Cells: Hydrogen is employed as a clean and efficient fuel source for energy generation.
-
Chemical Synthesis: Serves as a reducing agent in numerous chemical reactions.
Scientific Research:
-
Nuclear Fusion: The fusion of hydrogen isotopes (deuterium and tritium) holds immense potential as a clean and sustainable energy source.
-
Quantum Mechanics: Hydrogen’s simple atomic structure makes it a valuable model system for studying quantum mechanical principles.
Environmental Impact:
-
Renewable Energy: Hydrogen can be produced from renewable sources like solar and wind power, thus contributing to cleaner energy solutions.
-
Carbon Capture: Hydrogen may play a role in capturing and converting carbon dioxide, a greenhouse gas.
Conclusion: A Special Element Deserving Special Consideration
In conclusion, the question "Where is hydrogen in the periodic table?" doesn't have a single, definitive answer. Its unique properties, stemming from its simple atomic structure and its capacity to lose or gain electrons, make it an element that doesn't perfectly conform to any particular group. It's typically placed above the alkali metals, reflecting its ability to lose an electron, but its ability to gain an electron creates a similarity to the halogens. Ultimately, its unique position underscores its distinct chemical behavior and its immense importance across scientific research and industrial applications. It stands apart, a testament to the ever-evolving nature of chemical understanding and the surprising complexities hidden within even the simplest elements. The study of hydrogen's placement and behavior continuously expands our understanding of the periodic table and the fundamental laws governing chemistry. The multifaceted nature of this versatile element truly deserves further exploration.
Latest Posts
Latest Posts
-
What Is The Latent Heat Of Fusion
Mar 24, 2025
-
Earthworms And Leeches Belong To The Phylum
Mar 24, 2025
-
Classify The Below Solids As Amorphous Or Crystalline
Mar 24, 2025
-
Function Of Seminal Receptacles In Earthworm
Mar 24, 2025
-
What Is The Heat Of Hydration
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Where Is Hydrogen In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
