When Dissolved In Water All Acids Will
Juapaving
Mar 22, 2025 · 6 min read
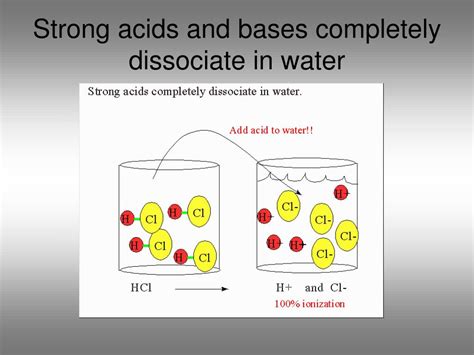
Table of Contents
When Dissolved in Water, All Acids Will... Produce Hydronium Ions!
The seemingly simple statement, "When dissolved in water, all acids will...", holds a fundamental truth about the nature of acids and their behavior in aqueous solutions. The complete answer is: When dissolved in water, all acids will donate a proton (H⁺) to a water molecule, forming hydronium ions (H₃O⁺). This process, known as protonation, is the defining characteristic of acids according to the Brønsted-Lowry acid-base theory. Let's delve deeper into this crucial concept, exploring the nuances of acid dissociation, the strength of acids, and the implications of hydronium ion formation.
Understanding Acid-Base Theories
Before diving into the specifics of hydronium ion formation, let's briefly review the major acid-base theories:
-
Arrhenius Theory: This is the simplest theory, defining acids as substances that produce hydrogen ions (H⁺) in water, and bases as substances that produce hydroxide ions (OH⁻) in water. While useful for many common acids and bases, it's limited as it doesn't account for acid-base reactions in non-aqueous solvents.
-
Brønsted-Lowry Theory: This broader theory defines acids as proton donors and bases as proton acceptors. This definition encompasses a wider range of substances and reactions, including those occurring without water as a solvent. This theory is central to understanding the formation of hydronium ions.
-
Lewis Theory: The most general theory, it defines acids as electron-pair acceptors and bases as electron-pair donors. While encompassing even more reactions, it’s less directly relevant to the specific question of hydronium ion formation in water.
For our discussion concerning the dissolution of acids in water, the Brønsted-Lowry theory provides the most pertinent framework.
The Formation of Hydronium Ions: A Detailed Look
When an acid dissolves in water, it interacts with water molecules. The crucial step is the transfer of a proton (H⁺) from the acid molecule to a water molecule. This proton transfer is facilitated by the polar nature of water, which has a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. The oxygen atom of the water molecule is attracted to the positively charged proton, accepting it to form a hydronium ion (H₃O⁺).
Let's illustrate this with a few examples:
-
Hydrochloric acid (HCl): A strong acid, HCl readily donates its proton to water:
HCl(aq) + H₂O(l) → H₃O⁺(aq) + Cl⁻(aq)The chloride ion (Cl⁻) is the conjugate base of HCl.
-
Acetic acid (CH₃COOH): A weak acid, acetic acid only partially donates its proton:
CH₃COOH(aq) + H₂O(l) ⇌ H₃O⁺(aq) + CH₃COO⁻(aq)The equilibrium arrows indicate that the reaction is reversible, and a significant portion of acetic acid remains undissociated in solution. The acetate ion (CH₃COO⁻) is the conjugate base of acetic acid.
-
Sulfuric acid (H₂SO₄): A strong diprotic acid, sulfuric acid can donate two protons in stepwise reactions:
H₂SO₄(aq) + H₂O(l) → H₃O⁺(aq) + HSO₄⁻(aq) HSO₄⁻(aq) + H₂O(l) ⇌ H₃O⁺(aq) + SO₄²⁻(aq)The first proton dissociation is essentially complete, while the second is only partially complete.
These examples demonstrate that the core process remains consistent: proton donation to water, resulting in hydronium ion formation. The extent of this donation, however, varies significantly depending on the strength of the acid.
Strong Acids vs. Weak Acids: The Extent of Dissociation
The key difference between strong and weak acids lies in the extent to which they dissociate in water.
-
Strong acids completely dissociate into their constituent ions in water. This means that virtually every acid molecule donates its proton to a water molecule, resulting in a high concentration of hydronium ions. Examples include HCl, HBr, HI, HNO₃, and H₂SO₄ (first proton).
-
Weak acids only partially dissociate in water. This means that only a fraction of the acid molecules donate their protons, resulting in a relatively low concentration of hydronium ions. The dissociation is an equilibrium process, meaning that both the undissociated acid and its conjugate base are present in significant concentrations. Examples include acetic acid (CH₃COOH), carbonic acid (H₂CO₃), and many organic acids.
The difference in dissociation is reflected in the acid dissociation constant (Ka). Strong acids have very large Ka values (meaning they dissociate extensively), while weak acids have small Ka values (meaning they dissociate to a lesser extent).
The Importance of Hydronium Ions
The formation of hydronium ions is crucial for understanding the acidic properties of solutions. The concentration of hydronium ions determines the pH of the solution. A lower pH indicates a higher concentration of hydronium ions, and thus a stronger acid. The pH scale is logarithmic, meaning that each whole number change represents a tenfold change in hydronium ion concentration.
Hydronium ions are responsible for many characteristic properties of acids, including:
-
Sour taste: The sour taste of acidic substances is due to the interaction of hydronium ions with taste receptors on the tongue.
-
Reaction with metals: Acids react with many metals, producing hydrogen gas and a metal salt. This reaction is driven by the high reactivity of hydronium ions.
-
Reaction with bases: Acids react with bases in neutralization reactions, forming water and a salt. This reaction involves the combination of hydronium ions with hydroxide ions (OH⁻) from the base.
-
Indicator color changes: Acid-base indicators change color depending on the pH of the solution, which is directly related to the hydronium ion concentration.
Polyprotic Acids: Donating Multiple Protons
Some acids, known as polyprotic acids, can donate more than one proton per molecule. Sulfuric acid (H₂SO₄) is a common example, as shown previously. The dissociation of polyprotic acids occurs in steps, with each step having its own acid dissociation constant (Ka). The first proton is usually easier to donate than subsequent protons.
Factors Affecting Acid Strength
Several factors influence the strength of an acid and therefore the extent of hydronium ion formation:
-
Electronegativity: Acids with more electronegative atoms attached to the acidic hydrogen tend to be stronger, as the electronegative atom pulls electron density away from the hydrogen, making it easier to donate.
-
Bond strength: Weaker bonds between the hydrogen and the rest of the molecule result in stronger acids, as the hydrogen is more readily released.
-
Resonance stabilization: If the conjugate base of the acid is stabilized by resonance, the acid will be stronger, as the formation of the conjugate base is more favorable.
-
Inductive effects: Electron-withdrawing groups attached to the molecule can increase the acidity, while electron-donating groups decrease it.
Conclusion: The Universal Truth of Hydronium Ion Formation
In conclusion, the statement "When dissolved in water, all acids will..." is completed by the crucial observation that all acids will donate a proton to water molecules, forming hydronium ions (H₃O⁺). While the extent of this proton donation varies considerably depending on the acid's strength, the fundamental process remains the same. Understanding this fundamental principle is crucial for grasping the behavior of acids in aqueous solutions and their roles in countless chemical and biological processes. The formation of hydronium ions defines acidity, dictates pH, and underpins many of the characteristic chemical reactions associated with acids. From the simplest monoprotic acids to complex polyprotic acids, the creation of hydronium ions remains a universal and defining characteristic of acid behavior in water.
Latest Posts
Latest Posts
-
What Is The Lcm Of 6 9 And 12
Mar 22, 2025
-
Which Number Is A Factor Of 18
Mar 22, 2025
-
Is The Square Root Of 18 A Rational Number
Mar 22, 2025
-
Why Are There More Herbivores Than Carnivores
Mar 22, 2025
-
How To Spell 8 In Words
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about When Dissolved In Water All Acids Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
