When Diluting An Acid With Water
Juapaving
Mar 09, 2025 · 6 min read
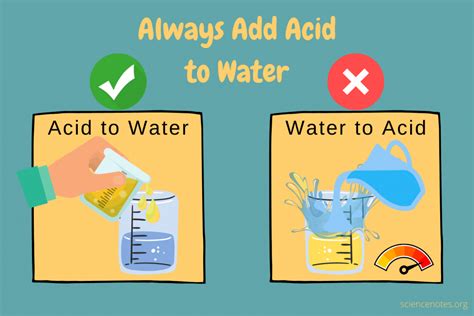
Table of Contents
When Diluting an Acid with Water: A Comprehensive Guide
Diluting acids is a fundamental procedure in many scientific and industrial settings. However, it's a process that demands meticulous care and a thorough understanding of the underlying principles. Improper dilution can lead to dangerous, even violent, reactions resulting in splashes, burns, and the release of harmful fumes. This comprehensive guide will delve into the crucial aspects of safely and effectively diluting an acid with water, emphasizing the "always add acid to water" rule and explaining the scientific reasoning behind it.
The Critical Importance of "Always Add Acid to Water"
The mantra of "always add acid to water, and never water to acid" is not merely a safety precaution; it's a fundamental principle rooted in the exothermic nature of acid dilution. When an acid dissolves in water, it releases a significant amount of heat. This process is called heat of dilution, or more generally, heat of solution. The amount of heat released varies depending on the concentration and type of acid.
The Exothermic Reaction: Understanding the Heat Generation
The heat generated during acid dilution stems from the strong interaction between acid molecules and water molecules. When acid molecules are added to water, they readily dissociate into ions (e.g., H⁺ and anions). This dissociation process is highly exothermic, meaning it releases energy in the form of heat. The stronger the acid, the more heat is released.
Consider the dilution of concentrated sulfuric acid (H₂SO₄) as a prime example. Sulfuric acid is a highly corrosive and viscous liquid. When water is added to concentrated sulfuric acid, the heat generated is intense enough to cause the water to boil violently. This boiling can lead to:
- Spattering: The boiling water can forcefully eject droplets of the corrosive acid mixture, causing burns and potential injuries.
- Steam Generation: The heat can create a substantial amount of steam, carrying acid droplets and potentially causing respiratory problems.
- Runaway Reaction: In extreme cases, the rapid heat generation can lead to a runaway reaction, where the temperature increases uncontrollably, potentially leading to a fire or explosion.
The Mitigating Effect of Adding Acid to Water
By contrast, when acid is slowly added to water, the heat generated is dispersed throughout a larger volume of water. The water acts as a heat sink, absorbing the released energy and preventing a sudden, dramatic temperature increase. This controlled dilution minimizes the risk of spattering, steam generation, and runaway reactions. The increased volume of water also dilutes the acid concentration, further reducing the hazard.
Safety Precautions: Beyond the Basic Rule
While the "add acid to water" rule is paramount, it's only one piece of the safety puzzle. Several other crucial precautions are necessary when handling acids:
1. Personal Protective Equipment (PPE)
Always wear appropriate PPE when working with acids, including:
- Safety goggles: To protect your eyes from splashes.
- Acid-resistant gloves: To protect your hands from chemical burns.
- Lab coat: To protect your clothing and skin from spills.
- Appropriate respiratory protection: In cases where fumes are generated.
2. Working in a Well-Ventilated Area
Acid dilution can produce fumes, especially with strong acids. Always perform the dilution in a well-ventilated area or under a fume hood to prevent inhalation of hazardous vapors.
3. Gradual Addition and Stirring
Add the acid slowly and gradually while constantly stirring the solution. This ensures that the heat is evenly distributed and prevents localized heating. Use a magnetic stirrer with a stirring bar for consistent and controlled mixing.
4. Use of Appropriate Glassware
Use heat-resistant glassware, such as borosilicate glass (Pyrex), to withstand the heat generated during dilution. Avoid using thin-walled glassware, which can crack under thermal stress.
5. Ice Bath (For Highly Exothermic Reactions)
For extremely exothermic dilutions, such as with concentrated sulfuric acid, consider performing the dilution in an ice bath. The ice bath will further help to control the temperature and prevent excessive heating.
6. Proper Disposal
After dilution, the acid solution needs proper disposal. Never pour diluted acid down the drain without consulting relevant safety guidelines and regulations. Follow the specific disposal procedures outlined by your institution or local authorities.
Different Acids: Varying Precautions
The intensity of the exothermic reaction varies significantly depending on the type and concentration of the acid. Here's a brief overview:
Concentrated Sulfuric Acid (H₂SO₄):
Requires extreme caution. The heat of dilution is exceptionally high. Always add the acid dropwise to a large volume of water, stirring constantly and using an ice bath.
Concentrated Nitric Acid (HNO₃):
Also highly exothermic. Follow the same precautions as with sulfuric acid: slow addition, constant stirring, and potentially an ice bath, particularly for high concentrations.
Concentrated Hydrochloric Acid (HCl):
While less exothermic than sulfuric or nitric acid, still requires careful dilution. Slow addition and constant stirring are essential.
Dilute Acids:
Dilution of already dilute acids presents less of a heat hazard, but safe laboratory practices should still be followed, including wearing appropriate PPE and performing the dilution in a well-ventilated area.
Understanding the Chemistry: A Deeper Dive
The exothermic nature of acid dilution is rooted in the strong solvation of ions. When an acid dissolves in water, the strong electrostatic interactions between the ions and water molecules release energy. This energy is manifested as heat. The enthalpy change (ΔH) associated with the dissolution of an acid is negative, indicating an exothermic process.
The strength of the acid influences the magnitude of the heat released. Strong acids, such as sulfuric and nitric acid, completely dissociate in water, leading to a greater release of heat compared to weak acids, which only partially dissociate.
Furthermore, the concentration of the acid directly affects the heat generated. Higher concentrations lead to more significant heat release. This is why it's crucial to add concentrated acids slowly and carefully.
Beyond the Laboratory: Industrial Applications
The principles of safe acid dilution are equally crucial in industrial settings. Large-scale acid dilutions often involve specialized equipment, such as automated dilution systems with temperature control and monitoring. These systems ensure controlled dilution, preventing hazardous events and maintaining safety. Strict safety protocols and training programs are essential in these environments.
Conclusion: Safety First
Diluting acids is a common procedure, but it's a process that requires strict adherence to safety protocols. The "always add acid to water" rule is not just a suggestion; it's a fundamental principle for preventing hazardous situations. Combining this rule with other safety precautions, such as using appropriate PPE, working in a well-ventilated area, and employing careful techniques like slow addition and constant stirring, significantly minimizes the risk of accidents and ensures a safe working environment. Remember, safety should always be the top priority when working with any chemical, especially strong acids. Always consult relevant safety data sheets (SDS) for detailed information and specific safety instructions related to the acids you are handling.
Latest Posts
Latest Posts
-
Rusting Iron Chemical Or Physical Change
Mar 09, 2025
-
Which Of The Following Is Not A Stage Of Mitosis
Mar 09, 2025
-
How To Find The Area And Perimeter Of A Triangle
Mar 09, 2025
-
Smallest Particle Of An Element That Retains Its Properties
Mar 09, 2025
-
How To Know If A Number Is Divisible By 6
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about When Diluting An Acid With Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
