What Number Is Iron On The Periodic Table
Juapaving
Mar 17, 2025 · 7 min read
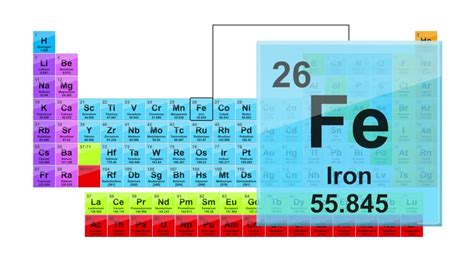
Table of Contents
What Number is Iron on the Periodic Table? Exploring the Properties and Significance of Element 26
Iron. The very word conjures images of strength, resilience, and perhaps even a touch of rust. But beyond its common connotations, iron holds a crucial place in the history of humanity and the fabric of our universe. Understanding its position on the periodic table—element number 26—is key to appreciating its significance. This article delves into the fascinating world of iron, exploring its atomic structure, properties, uses, and its fundamental role in various aspects of life and technology.
Iron's Atomic Structure and Position on the Periodic Table
The periodic table, a cornerstone of chemistry, arranges elements based on their atomic number, electron configuration, and recurring chemical properties. Iron, symbolized as Fe (from the Latin ferrum), sits proudly at atomic number 26. This number represents the number of protons in the atom's nucleus, defining its unique identity as iron. Its neutral atom also contains 26 electrons orbiting the nucleus, arranged in electron shells to achieve stability.
Electron Configuration and Chemical Behavior
The electron configuration of iron is [Ar] 3d<sup>6</sup> 4s<sup>2</sup>. This arrangement of electrons is crucial in determining iron's chemical reactivity. The two electrons in the 4s subshell are the first to be involved in chemical bonding, while the six electrons in the 3d subshell contribute to its variable oxidation states. This ability to exist in multiple oxidation states (primarily +2 and +3) accounts for iron's diverse chemical behavior and its ability to form a wide array of compounds.
Periodic Table Group and Period
Iron resides in Period 4 and Group 8 (or Group VIII) of the periodic table. Its placement in Period 4 indicates that it has four electron shells. Its position in Group 8, also known as the transition metals, highlights its characteristic properties, including variable oxidation states, the ability to form colored compounds, and catalytic activity.
Properties of Iron: A Strong and Versatile Element
Iron's position on the periodic table directly influences its physical and chemical properties, making it a remarkably versatile element with a wide range of applications.
Physical Properties
- Solid at Room Temperature: Iron exists as a solid under normal conditions, possessing a characteristic silvery-gray metallic luster when pure.
- High Melting and Boiling Points: Iron has a relatively high melting point (1538 °C) and boiling point (2862 °C), reflecting the strong metallic bonds between its atoms.
- Malleability and Ductility: Iron is both malleable (capable of being hammered into sheets) and ductile (capable of being drawn into wires), properties that are crucial for its use in various manufacturing processes.
- Ferromagnetism: This is perhaps iron's most distinctive property. Iron is strongly ferromagnetic, meaning it can be easily magnetized and retain its magnetism, a property essential to many technologies.
Chemical Properties
- Reactivity: Iron is a relatively reactive metal, readily reacting with oxygen (oxidation) to form iron oxides (rust) in the presence of moisture. This reactivity is both a benefit and a drawback, requiring protective measures in many applications.
- Variable Oxidation States: As mentioned earlier, iron can exist in multiple oxidation states, most commonly +2 (ferrous) and +3 (ferric). This contributes to the diversity of iron compounds and their varied applications.
- Formation of Alloys: Iron's ability to form alloys with other metals is critical to its widespread use. Steel, an alloy of iron and carbon, is a prime example, possessing enhanced strength and durability compared to pure iron.
- Catalysis: Iron and its compounds are used as catalysts in various industrial processes, facilitating chemical reactions without being consumed themselves.
The Significance of Iron: From Ancient Times to Modern Technology
Iron's significance transcends its mere presence on the periodic table; it plays a crucial role in many aspects of human civilization and the natural world.
Historical Significance: The Iron Age
The discovery and utilization of iron marked a pivotal moment in human history, ushering in the Iron Age. The ability to produce iron tools and weapons significantly advanced agricultural practices, warfare, and overall societal development. This transition reflects the profound impact of this element on the course of civilization.
Biological Significance: Essential for Life
Iron is an essential element for life. It serves as a vital component of hemoglobin, the protein in red blood cells responsible for oxygen transport throughout the body. Iron deficiency can lead to anemia, a condition characterized by fatigue and weakness. Furthermore, iron plays a crucial role in various other biological processes, highlighting its fundamental importance to living organisms.
Industrial Applications: A Cornerstone of Modern Industry
Iron and its alloys, particularly steel, are cornerstones of modern industry. Their strength, durability, and versatility make them indispensable in construction, manufacturing, transportation, and countless other sectors. From skyscrapers to automobiles to medical equipment, iron's presence is ubiquitous in our daily lives.
Technological Applications: Beyond Strength and Durability
Beyond its structural applications, iron finds use in various technologies. Its magnetic properties are exploited in electric motors, generators, and data storage devices. Iron-based catalysts are employed in various chemical processes, and iron compounds have applications in medicine, pigments, and more.
Iron Compounds and Their Diverse Uses
The versatility of iron is further highlighted by the diverse range of compounds it forms. These compounds exhibit a wide array of properties and applications.
Iron Oxides: From Pigments to Rust
Iron oxides, such as hematite (Fe₂O₃) and magnetite (Fe₃O₄), are abundant in nature and have numerous applications. Hematite is a major iron ore, while magnetite is a naturally occurring magnetic mineral. Iron oxides also serve as pigments in paints, cosmetics, and ceramics. However, iron oxide is also the main component of rust, which is the corrosion of iron.
Iron Sulfides: Natural Minerals and Industrial Applications
Iron sulfides, such as pyrite (FeS₂) also known as "fool's gold," are naturally occurring minerals. Pyrite, despite its appearance, is not gold. However, it does have certain industrial applications, such as the production of sulfuric acid.
Iron Chlorides: Industrial Chemicals and Water Treatment
Iron chlorides, like ferrous chloride (FeCl₂) and ferric chloride (FeCl₃), are important industrial chemicals used in water treatment and other industrial processes. Ferric chloride is an effective coagulant used to remove impurities from water.
Iron Carbonates: Minerals and Industrial Applications
Iron carbonates, like siderite (FeCO₃), are naturally occurring minerals. They are also used as iron supplements and in certain industrial applications.
Environmental Considerations: Rust and Recycling
While iron is essential for life and industry, its environmental impact needs careful consideration.
Rust and Corrosion: Economic and Environmental Costs
Rust, the oxidation of iron, leads to significant economic and environmental losses. Corrosion of iron structures can compromise safety and necessitate costly repairs. The environmental impact includes the release of iron ions into the environment, potentially affecting water quality and ecosystems.
Recycling Iron: Sustainable Practices
Recycling iron is a crucial strategy for mitigating its environmental impact. Recycling iron reduces the need for mining new iron ore, conserving natural resources and reducing energy consumption. Iron is highly recyclable and can be repeatedly melted and re-used with minimal loss of quality.
Conclusion: The Enduring Significance of Element 26
Iron's position at number 26 on the periodic table is a testament to its fundamental importance. From its role in the evolution of human civilization to its continued significance in modern technology and biological systems, iron's influence is profound and far-reaching. Understanding its atomic structure, properties, and applications is crucial to appreciating the multifaceted nature of this remarkable element. As we move towards a more sustainable future, embracing responsible iron production, utilization, and recycling will be critical to harnessing its benefits while mitigating its environmental impact. The story of iron, element 26, is a continuous narrative of innovation, adaptation, and the enduring power of a single element to shape the world around us.
Latest Posts
Latest Posts
-
Least Common Multiple Of 2 4 And 8
Mar 18, 2025
-
How Many Equal Sides Does An Isosceles Triangle Have
Mar 18, 2025
-
Full Form Of S I T
Mar 18, 2025
-
Which Number Is A Multiple Of 5
Mar 18, 2025
-
What Are The Advantages And Disadvantages Of A Democracy
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Number Is Iron On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
