What Makes Metal A Good Conductor
Juapaving
Mar 22, 2025 · 5 min read
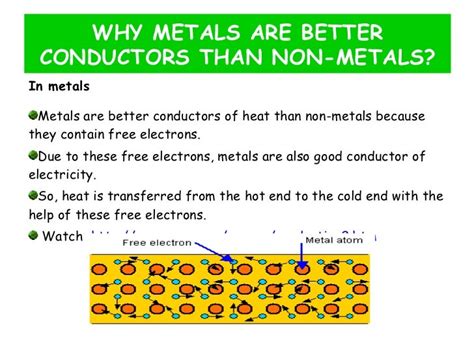
Table of Contents
What Makes Metal a Good Conductor of Electricity and Heat?
Metals are ubiquitous in our daily lives, from the smartphones in our pockets to the power grids that light our cities. This widespread use is largely due to their exceptional ability to conduct both electricity and heat. But what is it about the atomic structure and properties of metals that grants them this remarkable conductivity? This article delves deep into the fascinating world of metallic conduction, exploring the underlying mechanisms and factors influencing their conductive prowess.
The Sea of Electrons: The Key to Metallic Conductivity
The key to understanding metallic conductivity lies in their unique atomic structure. Unlike other materials, metals possess a distinctive arrangement of atoms and electrons known as the electron sea model. In this model, metal atoms are closely packed together in a lattice structure, but their valence electrons—the electrons in the outermost shell—are not tightly bound to individual atoms. Instead, these valence electrons are delocalized, forming a "sea" of freely moving electrons that are shared amongst all the atoms in the metallic lattice.
How the Electron Sea Enables Conduction:
This "sea" of delocalized electrons is the primary reason for metals' excellent conductivity. When an electric field is applied across a metal, these free electrons are readily accelerated by the field, resulting in a net flow of charge—that's electric current. The ease with which these electrons can move explains why metals are such efficient conductors of electricity. The higher the density of these free electrons, the greater the conductivity.
Similarly, heat conduction in metals is also explained by the electron sea model. When one part of a metal is heated, the kinetic energy of the electrons in that region increases. These energetic electrons then collide with neighboring electrons, transferring their energy throughout the metal. This rapid energy transfer across the electron sea explains the high thermal conductivity observed in metals.
Factors Affecting Metallic Conductivity:
While the electron sea model provides a basic understanding, several factors influence the actual conductivity of a metal:
1. Number of Valence Electrons:
The number of valence electrons an atom possesses directly affects the density of the electron sea. Metals with more valence electrons generally have higher electrical and thermal conductivity. For example, copper (with one valence electron) is a good conductor, but aluminum (with three valence electrons) exhibits even higher conductivity.
2. Atomic Arrangement and Crystal Structure:
The arrangement of atoms within the metallic lattice significantly impacts conductivity. A well-ordered, crystalline structure allows electrons to move more freely, leading to higher conductivity. Defects in the crystal structure, such as dislocations or impurities, can scatter electrons, hindering their movement and reducing conductivity. This is why highly pure metals generally exhibit better conductivity than alloys (mixtures of metals).
3. Temperature:
Temperature plays a crucial role in metallic conductivity. As temperature increases, the atoms within the metallic lattice vibrate more vigorously. This increased atomic vibration leads to more frequent collisions between electrons and atoms, scattering the electrons and reducing their ability to flow freely. This is why the electrical and thermal conductivity of metals generally decreases with increasing temperature.
4. Impurities and Alloying:
The presence of impurities or alloying elements significantly affects metallic conductivity. Impurities act as scattering centers for electrons, impeding their movement and reducing conductivity. Alloying, the process of mixing different metals, can either increase or decrease conductivity depending on the specific metals involved and their concentration. Some alloying elements can enhance conductivity, while others can significantly reduce it.
5. Grain Size and Boundaries:
The size and distribution of grains (crystalline regions) within a metal also influence conductivity. Grain boundaries act as barriers to electron flow, scattering electrons and reducing conductivity. Smaller grain sizes, with more grain boundaries, generally lead to lower conductivity compared to larger grain sizes. This is why techniques like grain refinement are employed to enhance the properties of metals.
Different Types of Metals and Their Conductivity:
The conductivity of metals varies widely depending on their atomic structure and the factors discussed above. Some metals are exceptionally good conductors, while others exhibit lower conductivity.
Excellent Conductors:
- Silver (Ag): Silver possesses the highest electrical and thermal conductivity of all metals. Its high conductivity is due to the single valence electron and its relatively low atomic weight.
- Copper (Cu): Copper is a widely used conductor due to its excellent conductivity, relatively low cost, and ease of fabrication.
- Gold (Au): Gold is another excellent conductor, prized for its corrosion resistance and stability.
- Aluminum (Al): Aluminum offers a high conductivity-to-weight ratio, making it suitable for applications where weight is a critical factor.
Moderately Good Conductors:
- Iron (Fe): Iron is a relatively good conductor, but its conductivity is lower than that of silver, copper, or aluminum.
- Nickel (Ni): Nickel has a moderate conductivity and is often used in alloys to improve their properties.
Poor Conductors (Compared to the above):
- Lead (Pb): Lead is a relatively poor conductor compared to the metals mentioned above.
- Mercury (Hg): Mercury is a liquid metal with relatively low conductivity.
Applications of Metallic Conductivity:
The remarkable conductive properties of metals have led to their extensive use in a wide range of applications:
- Electrical Wiring: Copper and aluminum are widely used in electrical wiring due to their high conductivity and relatively low cost.
- Power Transmission Lines: High-voltage power transmission lines often use aluminum conductors due to their lightweight and high conductivity.
- Electronic Components: Metals such as copper, gold, and aluminum are used extensively in electronic components like integrated circuits and printed circuit boards.
- Heat Exchangers: Metals like copper and aluminum are used in heat exchangers due to their excellent thermal conductivity.
- Cooking Utensils: Metals such as copper, stainless steel, and aluminum are used in cookware due to their ability to efficiently transfer heat.
Conclusion:
The exceptional conductivity of metals is a direct consequence of their unique atomic structure, specifically the presence of a sea of delocalized valence electrons. This electron sea allows for the easy movement of charge and energy, resulting in high electrical and thermal conductivity. While the electron sea model provides a fundamental understanding, various factors such as the number of valence electrons, crystal structure, temperature, impurities, and grain size influence the actual conductivity of a metal. Understanding these factors is crucial for selecting appropriate metals for various applications, from electrical wiring to advanced electronic devices and heat transfer systems. The continued study and development of metallic materials with enhanced conductivity remain a significant area of research with implications for various technological advancements.
Latest Posts
Latest Posts
-
What Is The Chemical Formula Of Gold
Mar 22, 2025
-
Who Was The Father Of Renaissance
Mar 22, 2025
-
Is Melting Ice Cream A Physical Change
Mar 22, 2025
-
What Mountain Range Divides Europe From Asia
Mar 22, 2025
-
Sum Of Interior Angles Of A Dodecagon
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Makes Metal A Good Conductor . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
