What Is The Specific Heat Of Liquid Water
Juapaving
Apr 01, 2025 · 5 min read
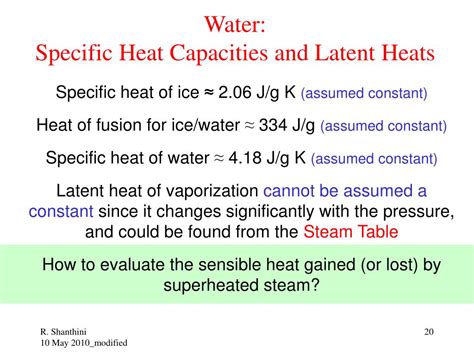
Table of Contents
What is the Specific Heat of Liquid Water? A Deep Dive
The specific heat of liquid water is a fundamental concept in physics and chemistry, playing a crucial role in various natural phenomena and technological applications. Understanding its value and implications is key to grasping many aspects of our world. This article delves deep into the specific heat of liquid water, exploring its definition, measurement, significance, and the factors influencing its value.
Defining Specific Heat Capacity
Before focusing on water, let's clarify the term "specific heat capacity." Specific heat capacity, often shortened to specific heat, is the amount of heat required to raise the temperature of one unit of mass of a substance by one degree Celsius (or one Kelvin). It's a measure of a substance's resistance to temperature change. A substance with a high specific heat requires a lot of energy to increase its temperature, while a substance with a low specific heat requires less. The unit for specific heat is typically Joules per kilogram per Kelvin (J/kg·K) or calories per gram per degree Celsius (cal/g·°C).
The Remarkable Specific Heat of Water
Water boasts an exceptionally high specific heat capacity compared to many other common substances. Its value is approximately 4.186 joules per gram per degree Celsius (J/g·°C) or 1 calorie per gram per degree Celsius (cal/g·°C). This means it takes 4.186 Joules of energy to raise the temperature of 1 gram of water by 1°C. This seemingly simple number has profound consequences for the planet and our lives.
Why is Water's Specific Heat So High?
This unusually high specific heat is due to the unique properties of water molecules and their interactions.
-
Hydrogen Bonding: Water molecules are polar, meaning they have a slightly positive end and a slightly negative end. This polarity allows them to form strong hydrogen bonds with each other. These bonds require significant energy to break, contributing to water's high specific heat. When heat is added, much of the energy goes into breaking these bonds rather than directly increasing the kinetic energy (and thus temperature) of the molecules.
-
Molecular Structure and Vibrational Modes: The bent molecular structure of water and its various vibrational modes (stretching, bending) also contribute to the energy absorption capacity. These vibrational modes absorb significant amounts of energy, further increasing the specific heat.
-
Intermolecular Forces: The strong intermolecular forces between water molecules require significant energy to overcome, further contributing to the high specific heat.
The Significance of Water's High Specific Heat
The high specific heat of water has numerous vital implications:
1. Climate Regulation:
Water's high specific heat plays a crucial role in moderating Earth's climate. Large bodies of water like oceans and lakes act as massive heat reservoirs, absorbing vast amounts of solar energy without experiencing drastic temperature fluctuations. This prevents extreme temperature swings, creating a more stable and habitable climate. Coastal regions, for instance, generally have milder temperatures than inland areas because of the moderating effect of the ocean.
2. Temperature Stability in Aquatic Ecosystems:
The high specific heat of water is essential for maintaining stable temperatures in aquatic ecosystems. This stability is crucial for the survival of aquatic organisms, as large temperature fluctuations can be detrimental. This stability allows for a diverse range of species to thrive in aquatic environments.
3. Biological Importance:
Water's high specific heat is critical for maintaining stable body temperatures in living organisms. Since our bodies are largely composed of water, our internal temperature remains relatively constant despite fluctuations in the external environment. This is crucial for proper metabolic function and overall health.
4. Industrial Applications:
The high specific heat of water makes it an excellent coolant in various industrial applications. It's commonly used in power plants, manufacturing processes, and other industries where efficient heat transfer is required. Its ability to absorb large amounts of heat without significant temperature increase makes it a highly effective cooling agent.
5. Meteorological Effects:
Water's high specific heat influences weather patterns. The slow heating and cooling of water bodies affect air temperature and humidity, influencing the formation of clouds, precipitation, and wind patterns.
Factors Affecting Water's Specific Heat
While the specific heat of water is relatively constant under standard conditions, several factors can subtly influence its value:
-
Temperature: The specific heat of water varies slightly with temperature. While the difference is relatively small within the typical range of liquid water (0°C to 100°C), it's measurable and important in precise calculations. The specific heat generally increases slightly with increasing temperature.
-
Pressure: Pressure also has a minor effect on the specific heat of water. The influence is generally more pronounced at higher pressures.
-
Salinity: The presence of dissolved salts (like in seawater) can slightly decrease the specific heat of water. The extent of this decrease depends on the salinity concentration.
-
Isotopic Composition: The isotopic composition of water (the ratio of different isotopes of hydrogen and oxygen) can also influence its specific heat. Heavy water (containing deuterium) has a slightly different specific heat compared to ordinary water.
Measuring the Specific Heat of Water
The specific heat of water can be experimentally determined using calorimetry. A calorimeter is a device designed to measure heat transfer. A common method involves heating a known mass of water and measuring the temperature change resulting from the addition of a known amount of heat. By applying the formula:
Q = mcΔT
where:
- Q = heat energy transferred (in Joules)
- m = mass of water (in grams or kilograms)
- c = specific heat of water (what we are trying to determine)
- ΔT = change in temperature (in °C or K)
we can calculate the specific heat of water. However, precise measurements require careful consideration of heat losses to the surroundings and other experimental errors.
Conclusion
The specific heat of liquid water, a seemingly simple physical property, has far-reaching implications across various scientific disciplines and practical applications. Its exceptionally high value, stemming from the unique properties of water molecules, is crucial for moderating Earth's climate, maintaining stable aquatic ecosystems, supporting life processes, and facilitating industrial applications. Understanding this fundamental property is essential for comprehending numerous aspects of our natural world and harnessing its power for technological advancements. Further research continues to refine our understanding of the factors influencing water's specific heat and its precise value under varying conditions.
Latest Posts
Latest Posts
-
Collection Of Nerve Cell Bodies Outside The Cns
Apr 02, 2025
-
What Do Nitrification And Denitrification Have In Common
Apr 02, 2025
-
What Is The Lowest Common Multiple Of 3 And 7
Apr 02, 2025
-
How To Find A Supplementary Angle
Apr 02, 2025
-
How Can The Strength Of An Electromagnet Be Increased
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Specific Heat Of Liquid Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
