What Is The Solvent In Air
Juapaving
Mar 20, 2025 · 6 min read
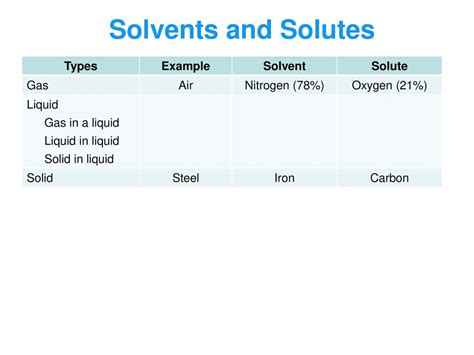
Table of Contents
What is the Solvent in Air? Understanding Air's Composition and Behavior
Air, the invisible essence surrounding us, is far from a simple entity. While often perceived as a single substance, air is actually a complex mixture of various gases, with one acting as the dominant solvent: nitrogen. This article delves into the composition of air, explains why nitrogen serves as the solvent, explores the concept of solubility in gaseous mixtures, and discusses the implications of this understanding.
The Composition of Air: More Than Just Oxygen
The Earth's atmosphere isn't a homogeneous blob of gas. It's a dynamic mixture, primarily composed of:
- Nitrogen (N₂): Approximately 78% of the Earth's atmosphere is nitrogen. This colorless, odorless, and relatively inert gas plays a crucial role as the solvent.
- Oxygen (O₂): Around 21% of the atmosphere is oxygen, essential for respiration in most living organisms. While vital, oxygen's concentration is significantly lower than nitrogen's.
- Argon (Ar): Constituting about 0.93%, argon is a noble gas, known for its inert nature.
- Other Gases: The remaining fraction (less than 1%) comprises trace gases like carbon dioxide (CO₂), neon (Ne), helium (He), methane (CH₄), krypton (Kr), hydrogen (H₂), nitrous oxide (N₂O), and xenon (Xe). The concentration of these trace gases can vary depending on location and factors like industrial activity and natural processes.
Water Vapor: A Variable Component
It's crucial to note that the above percentages generally refer to dry air. The atmosphere also contains a variable amount of water vapor (H₂O), which can range from near zero to several percent, depending on factors like temperature, humidity, and altitude. Water vapor significantly influences the properties of air, including its density and its capacity to dissolve other substances.
Nitrogen: The Dominant Solvent in Air
While the term "solvent" typically evokes images of liquids dissolving solids or other liquids, the principle extends to gaseous mixtures. In air, nitrogen, due to its abundance, acts as the primary solvent. This means other gases in the atmosphere are essentially "dissolved" within the nitrogen matrix.
Understanding Solubility in Gaseous Mixtures
Solubility in gases differs from solubility in liquids. It's primarily governed by partial pressures. Each gas in a mixture exerts its own partial pressure, which is proportional to its concentration. The total pressure of the air is the sum of all the partial pressures.
Henry's Law provides a fundamental understanding of gas solubility: the amount of gas dissolved in a liquid (or, in this case, in the gaseous mixture of air) is directly proportional to the partial pressure of that gas above the liquid. While this law is strictly for gas dissolving into a liquid, the concept of partial pressures and their proportionality to concentration is still relevant for explaining the distribution of gases within the air.
Therefore, because nitrogen has the highest partial pressure in the atmosphere, it acts as the solvent, and other gases are distributed within this dominant gaseous phase.
The Role of Partial Pressures in Air's Behavior
The concept of partial pressures is paramount in understanding how different components of air interact and behave:
-
Oxygen Uptake: Our respiratory systems don't extract oxygen directly from the "total air" but from the partial pressure of oxygen in the air we inhale. This partial pressure drives the diffusion of oxygen across the lung membranes into the bloodstream. Similarly, the partial pressure of carbon dioxide dictates its release from the blood into the lungs for exhalation.
-
Altitude Effects: At higher altitudes, the total atmospheric pressure decreases, leading to a decrease in the partial pressures of all gases, including oxygen. This explains why breathing becomes more difficult at high altitudes—there's less oxygen available per breath.
-
Atmospheric Pollution: Pollutants released into the atmosphere mix with the existing gases, adding to their respective partial pressures. Understanding these partial pressures is crucial for assessing the impact of pollutants and predicting their dispersion patterns.
-
Weather Phenomena: The partial pressure of water vapor is directly related to humidity. Changes in water vapor partial pressure influence cloud formation, precipitation, and other weather events.
Implications of Nitrogen's Role as the Solvent
Understanding nitrogen's role as the solvent in air has significant implications across diverse fields:
-
Environmental Science: Models for atmospheric pollution dispersion, climate change prediction, and ozone depletion all rely on accurately calculating and understanding the partial pressures of various gases in the atmosphere.
-
Aerospace Engineering: The design of aircraft and spacecraft requires considering the effects of changing atmospheric pressure and composition at different altitudes. Accurate predictions rely on understanding the behavior of the gaseous mixture, with nitrogen as the predominant component.
-
Medicine: Understanding gas solubility and partial pressure is essential for designing respiratory therapies, such as oxygen administration and managing conditions related to altitude sickness.
-
Industrial Processes: Various industrial processes, such as the production of certain chemicals or the operation of combustion engines, require a thorough understanding of the behavior of gases under various pressure and concentration conditions, with nitrogen's role as the solvent influencing the outcome and efficiency.
Beyond the Basics: Factors Affecting Air Composition
The composition of air is not static. Various factors influence its composition:
-
Geographic Location: Air composition can vary geographically due to factors like industrial emissions, volcanic activity, and proximity to oceans or forests.
-
Altitude: The composition of air changes with altitude. The concentration of certain gases, such as nitrogen and oxygen, decreases with increasing altitude, while others, like ozone, may have higher concentrations at specific altitudes.
-
Time of Day: Daily variations can occur due to changes in solar radiation, temperature, and biological activity. For example, carbon dioxide levels typically rise during the night and decrease during the day due to plant photosynthesis.
-
Season: Seasonal changes in temperature and weather patterns influence air composition, including the amount of water vapor and pollutants.
Conclusion: Air – A Dynamic Gaseous Solution
In conclusion, air is not merely a single gas; it's a dynamic and complex mixture with nitrogen playing the crucial role of the solvent. This understanding is fundamentally based on the principles of partial pressures and gas solubility. Understanding air's composition and the role of nitrogen as the solvent is critical for numerous scientific disciplines and practical applications, ranging from environmental monitoring and pollution control to aerospace engineering and medical treatments. As our understanding of atmospheric science continues to evolve, so too will our appreciation for the intricate interactions within this seemingly simple gaseous solution that sustains life on Earth. Further research into the subtle nuances of air composition and its responses to environmental changes will be crucial for addressing global challenges such as climate change and air quality management. The invisible world of atmospheric chemistry holds many more secrets waiting to be unveiled.
Latest Posts
Latest Posts
-
Mass Of An Electron In Kilograms
Mar 20, 2025
-
Which Are Not Considered Greenhouse Gases
Mar 20, 2025
-
How Many Minutes Is In 4 Hours
Mar 20, 2025
-
Things That Start With A X
Mar 20, 2025
-
What Is The Smallest Unit That Makes Up Matter
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is The Solvent In Air . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
