What Is The Role Of The Electrode
Juapaving
Mar 06, 2025 · 6 min read
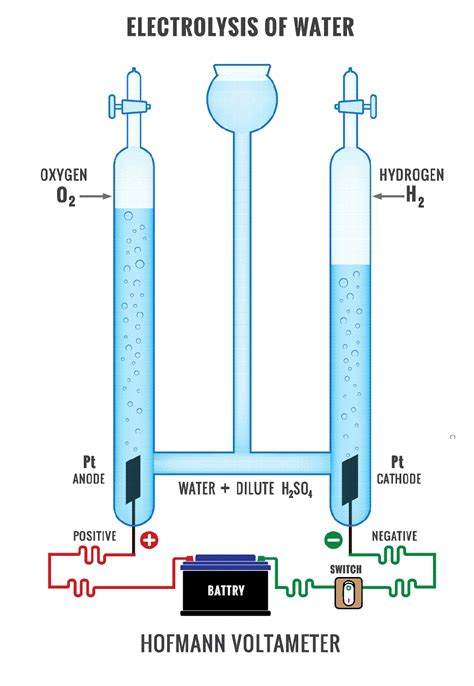
Table of Contents
What is the Role of the Electrode? A Deep Dive into Electrochemical Processes
Electrodes are fundamental components in a vast array of technologies, from powering our smartphones to diagnosing medical conditions. Understanding their role is crucial to grasping the principles behind batteries, fuel cells, sensors, and many other electrochemical devices. This article delves deep into the multifaceted role of electrodes, exploring their function, types, materials, and applications across various fields.
The Fundamental Role of Electrodes: Facilitating Electron Transfer
At its core, the role of an electrode is to facilitate the transfer of electrons between an external circuit and an electrolyte (a solution containing ions). This electron transfer is the essence of electrochemical reactions, which involve the conversion of chemical energy into electrical energy (or vice versa). The electrode acts as an interface, allowing electrons to flow into or out of the electrolyte, driving the chemical reactions that power countless devices.
Two Key Electrode Types: Anode and Cathode
Electrodes are categorized into two types based on their function in an electrochemical cell:
-
Anode: The anode is where oxidation occurs. Oxidation is a process where a substance loses electrons. In essence, the anode supplies electrons to the external circuit. Think of it as the "electron source."
-
Cathode: The cathode is where reduction occurs. Reduction is a process where a substance gains electrons. The cathode receives electrons from the external circuit. Think of it as the "electron sink."
The terms anode and cathode are not inherently positive or negative. Their polarity depends entirely on the specific electrochemical cell and the direction of electron flow. In a battery discharging, the anode is negative and the cathode is positive. However, during charging, the polarities reverse.
Electrode Materials: A Diverse Landscape of Properties
The choice of electrode material is critical and dictates the performance characteristics of the electrochemical device. The ideal electrode material needs to possess several key properties:
-
High Electrical Conductivity: The electrode must efficiently conduct electrons to and from the external circuit, minimizing internal resistance. Materials like metals (e.g., platinum, gold, copper) and conductive polymers are often used.
-
Electrochemical Stability: The electrode material must withstand the chemical environment of the electrolyte without undergoing significant degradation or corrosion. This is particularly important in harsh conditions, such as high temperatures or highly corrosive electrolytes.
-
Good Catalytic Activity (in some cases): For many applications, the electrode needs to catalyze the electrochemical reactions, speeding up the rate of electron transfer. This is especially true in fuel cells and electrolyzers, where the reaction rates significantly impact efficiency. Platinum is a widely used catalyst due to its excellent catalytic activity.
-
Porosity (often desired): A high surface area is often advantageous, especially in applications requiring high reaction rates. Porous electrodes provide a large surface area for the electrochemical reaction to occur, improving efficiency and power density.
Examples of Electrode Materials:
-
Metals: Platinum, gold, silver, nickel, copper are common choices due to their high conductivity and electrochemical stability. However, their cost and scarcity can be limiting factors.
-
Carbon-based Materials: Graphite, carbon nanotubes, and graphene are increasingly popular due to their excellent conductivity, low cost, and abundance. They are used in various applications, including batteries and supercapacitors.
-
Metal Oxides: Materials like RuO2 and IrO2 are employed as electrocatalysts in various electrochemical processes.
-
Conductive Polymers: These polymers exhibit electrical conductivity and can be tailored with specific functionalities, making them suitable for various applications.
-
Composite Materials: Combining different materials can optimize the properties of the electrode, leading to enhanced performance. For example, a composite material might combine a conductive carbon backbone with a metal oxide catalyst to create a high-performance electrode.
Applications Across Diverse Fields: The Ubiquitous Electrode
Electrodes play a crucial role across a wide range of applications, including:
1. Batteries: Powering Our World
In batteries, electrodes are essential for storing and releasing electrical energy. The anode undergoes oxidation (releasing electrons), while the cathode undergoes reduction (accepting electrons). The flow of electrons through the external circuit constitutes the electrical current. Different battery chemistries utilize various electrode materials, each with its unique characteristics in terms of energy density, power density, and cycle life. Examples include Lithium-ion batteries (using graphite and lithium metal oxides), lead-acid batteries, and nickel-metal hydride batteries.
2. Fuel Cells: Clean Energy Conversion
Fuel cells convert chemical energy directly into electrical energy through electrochemical reactions. The anode oxidizes a fuel (e.g., hydrogen), while the cathode reduces an oxidant (e.g., oxygen). Electrodes play a critical role in catalyzing these reactions, ensuring efficient energy conversion. Platinum is a frequently used catalyst in fuel cells due to its excellent catalytic activity towards hydrogen oxidation and oxygen reduction.
3. Sensors: Detecting Chemical and Biological Species
Electrodes form the basis of many electrochemical sensors. These sensors utilize the changes in electrical signals (current, potential) resulting from electrochemical reactions to detect the presence and concentration of specific chemical or biological species. Examples include pH sensors, ion-selective electrodes, glucose sensors, and biosensors.
4. Electrolysis: Generating Hydrogen and Other Chemicals
Electrolysis uses an electric current to drive chemical reactions. The electrodes play a critical role in facilitating the oxidation and reduction reactions, producing desired chemicals. Electrolysis is used in the production of hydrogen from water, chlorine from brine, and various other industrial processes.
5. Electroplating and Electropolishing: Surface Modification
Electrodes are fundamental in electroplating and electropolishing processes. In electroplating, a metal is deposited onto a substrate using an electric current, improving its properties (e.g., corrosion resistance, appearance). Electropolishing removes surface imperfections from a metal using an electrochemical process.
6. Biomedical Applications: Diagnostics and Treatment
Electrodes are used in various biomedical applications. In electroencephalography (EEG), electrodes monitor brain activity. In electrocardiography (ECG), they measure heart activity. Electrodes are also employed in pacemakers, defibrillators, and other implantable medical devices.
Future Trends in Electrode Technology
Research and development in electrode technology are continuously advancing, focusing on:
-
Development of Novel Materials: The search for new materials with improved properties (e.g., higher conductivity, better catalytic activity, enhanced stability) is ongoing. This includes exploring 2D materials, metal-organic frameworks (MOFs), and other advanced materials.
-
Improved Electrode Design: Optimizing electrode design to enhance surface area, reduce internal resistance, and improve mass transport is crucial. This includes exploring hierarchical porous structures, 3D printing techniques, and other advanced fabrication methods.
-
Integration with Nanotechnology: Nanotechnology offers opportunities to create highly efficient and miniaturized electrodes with enhanced properties.
Conclusion: The Indispensable Role of Electrodes
Electrodes are indispensable components in a vast array of electrochemical devices and technologies. Their role in facilitating electron transfer is central to the functioning of batteries, fuel cells, sensors, and many other applications. Ongoing research and development in electrode materials and design will continue to drive innovation across various fields, shaping the future of energy storage, clean energy conversion, and advanced sensing technologies. The continued understanding and improvement of electrode technology will undoubtedly be crucial in addressing some of the most pressing challenges facing humanity, from climate change to improved healthcare.
Latest Posts
Latest Posts
-
What Is The Lcm Of 3 8
Mar 06, 2025
-
Is A Sound Wave A Transverse Wave
Mar 06, 2025
-
What Is The Electron Configuration For Magnesium
Mar 06, 2025
-
What Is The Common Factor Of 12 And 36
Mar 06, 2025
-
Which Organelles Are Found Only In Plant Cells
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about What Is The Role Of The Electrode . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
