What Is The Electron Configuration For Magnesium
Juapaving
Mar 06, 2025 · 6 min read
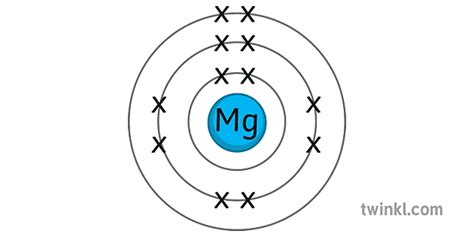
Table of Contents
What is the Electron Configuration for Magnesium? A Deep Dive into Atomic Structure
Magnesium, a vital element for both human health and numerous industrial applications, holds a fascinating place in the periodic table. Understanding its electron configuration is key to unlocking its unique properties and behaviors. This article will delve deep into the electron configuration of magnesium, explaining the underlying principles, its significance, and exploring related concepts.
Understanding Electron Configuration
Before diving into magnesium specifically, let's establish a foundational understanding of electron configuration. An electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement dictates how an atom will interact with other atoms, influencing its chemical reactivity and bonding capabilities. It follows specific rules based on the principles of quantum mechanics.
The Aufbau Principle and Hund's Rule
Two fundamental principles govern electron configuration:
-
The Aufbau Principle: This principle states that electrons fill atomic orbitals starting with the lowest energy levels and progressively moving to higher energy levels. Think of it like filling a building – you start on the ground floor before moving to higher floors.
-
Hund's Rule: This rule dictates that electrons will individually occupy each orbital within a subshell before pairing up. Imagine each orbital as a seat in a bus; each person (electron) will take a separate seat before sharing a seat with another person. This minimizes electron-electron repulsion.
Electron Shells, Subshells, and Orbitals
Understanding the terminology is crucial:
-
Electron Shells (Principal Energy Levels): These are the main energy levels, denoted by the principal quantum number (n), where n = 1, 2, 3, and so on. Shells closer to the nucleus have lower energy.
-
Subshells (Sublevels): Within each shell, there are subshells designated by letters: s, p, d, and f. Each subshell has a specific number of orbitals.
-
Orbitals: These are regions of space within a subshell where there's a high probability of finding an electron. Each orbital can hold a maximum of two electrons with opposite spins (Pauli Exclusion Principle).
- s subshell: Contains one orbital (holds up to 2 electrons)
- p subshell: Contains three orbitals (holds up to 6 electrons)
- d subshell: Contains five orbitals (holds up to 10 electrons)
- f subshell: Contains seven orbitals (holds up to 14 electrons)
Determining the Electron Configuration of Magnesium (Mg)
Magnesium (Mg) has an atomic number of 12, meaning it has 12 protons and 12 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle and Hund's rule.
The order of filling orbitals is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, and so on. However, there are exceptions to this rule, particularly with transition metals.
Following the Aufbau principle, the electron configuration for magnesium is:
1s² 2s² 2p⁶ 3s²
Let's break it down:
- 1s²: The first shell (n=1) contains the s subshell, which holds two electrons.
- 2s²: The second shell (n=2) contains the s subshell, which holds two electrons.
- 2p⁶: The second shell (n=2) also contains the p subshell, which can hold up to six electrons; in magnesium, it's fully occupied.
- 3s²: The third shell (n=3) contains the s subshell, which holds the remaining two electrons.
Significance of Magnesium's Electron Configuration
The electron configuration of magnesium (1s² 2s² 2p⁶ 3s²) is highly significant because it directly impacts its chemical properties:
-
Valence Electrons: The outermost electrons, those in the 3s orbital, are called valence electrons. Magnesium has two valence electrons. These electrons are involved in chemical bonding.
-
Reactivity: Magnesium's two valence electrons readily participate in chemical reactions. It tends to lose these two electrons to achieve a stable, filled outer shell configuration similar to noble gases (octet rule). This makes it highly reactive, particularly with nonmetals like oxygen and chlorine.
-
Ionic Bonding: Magnesium's tendency to lose two electrons leads to the formation of Mg²⁺ ions. This ionic bonding is the basis for many magnesium compounds, such as magnesium oxide (MgO) and magnesium chloride (MgCl₂).
-
Metallic Bonding: Magnesium's electron configuration also contributes to its metallic bonding. The valence electrons are delocalized, forming a "sea" of electrons that holds the positively charged magnesium ions together. This accounts for magnesium's properties like malleability, ductility, and good electrical conductivity.
Magnesium's Role in Biology and Industry
Magnesium's unique properties, stemming directly from its electron configuration, make it vital in various biological and industrial processes:
Biological Significance:
-
Enzyme Activation: Magnesium ions are essential cofactors for numerous enzymes involved in crucial metabolic processes, including DNA replication, protein synthesis, and ATP production.
-
Muscle Function: Magnesium plays a critical role in muscle contraction and relaxation.
-
Nerve Transmission: It contributes to the proper functioning of nerve impulses.
-
Bone Health: Magnesium is a crucial component of bone structure.
A deficiency in magnesium can lead to various health issues, highlighting its essential role in maintaining bodily functions.
Industrial Applications:
-
Alloying Agent: Magnesium's lightweight nature and ability to form strong alloys make it invaluable in the aerospace and automotive industries.
-
Structural Material: Its strength-to-weight ratio is exploited in various lightweight structural components.
-
Reducing Agent: Magnesium's reactivity is utilized in various metallurgical processes as a reducing agent.
-
Grignard Reagents: Organomagnesium compounds, known as Grignard reagents, are extensively used in organic synthesis.
Exploring Further: Excited States and Ionization Energy
While the ground state electron configuration described above is the most stable state for magnesium, it can also exist in excited states. An excited state occurs when an electron absorbs energy and jumps to a higher energy level. These excited states are less stable and will eventually return to the ground state, often emitting energy in the form of light.
Another crucial concept related to electron configuration is ionization energy. Ionization energy is the energy required to remove an electron from an atom or ion. Magnesium's low ionization energies for its two valence electrons reflect its tendency to lose these electrons, forming the stable Mg²⁺ ion.
Conclusion: The Importance of Understanding Electron Configuration
The electron configuration of magnesium, 1s² 2s² 2p⁶ 3s², is not simply an abstract concept; it's the fundamental basis for understanding magnesium's properties, reactivity, and its crucial roles in biology and industry. By grasping this configuration, we gain insight into the behavior of the element at the atomic level, which has far-reaching implications across multiple scientific disciplines. The principles discussed here — the Aufbau principle, Hund's rule, and the concepts of valence electrons, ionization energy, and excited states—are fundamental to comprehending the behavior of all elements in the periodic table. A deeper understanding of these concepts opens doors to more advanced studies in chemistry, physics, and materials science.
Latest Posts
Latest Posts
-
3 Of 16 Is What Percent
Mar 06, 2025
-
Least Common Multiple For 5 And 6
Mar 06, 2025
-
Is A Archaebacteria Multicellular Or Unicellular
Mar 06, 2025
-
What Is The Factorization Of 8
Mar 06, 2025
-
How Many Sides Do Octagons Have
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Magnesium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
