What Is The Positively Charged Particle Of An Atom
Juapaving
Mar 16, 2025 · 6 min read
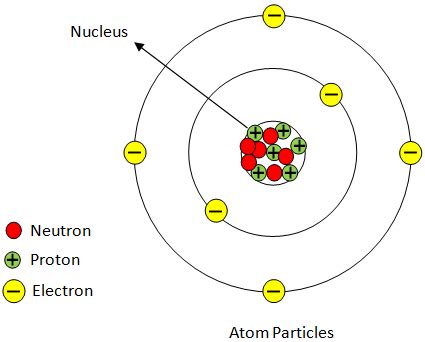
Table of Contents
What is the Positively Charged Particle of an Atom? A Deep Dive into Protons
The atom, the fundamental building block of matter, is a fascinating microcosm of physics. Understanding its constituents is crucial to grasping the nature of the universe itself. While often simplified in introductory science, the atom's structure is far more nuanced and complex than many realize. This article delves into the positively charged particle of an atom: the proton, exploring its properties, its role in atomic structure, and its significance in various scientific fields.
Understanding the Atom's Structure: A Three-Part Harmony
Before focusing solely on the proton, it's essential to establish a basic understanding of the atom's overall structure. Atoms are comprised of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus.
- Neutrons: Neutral particles (no charge) also located in the atom's nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The nucleus, the atom's central core, contains both protons and neutrons. This dense region accounts for nearly all of the atom's mass, while the electrons, though numerous, contribute minimally to the overall mass. The arrangement of these particles determines an atom's properties and its behavior in chemical reactions.
The Proton: A Closer Look at the Positive Charge
The proton, denoted by the symbol p⁺ or p, is a fundamental subatomic particle with a positive electric charge equal in magnitude but opposite in sign to that of an electron. This positive charge is what distinguishes it from neutrons and is crucial to the overall stability and interactions of atoms.
Properties of Protons: Key Characteristics
Let's explore some of the key properties that define a proton:
- Charge: +1 elementary charge (approximately 1.602 × 10⁻¹⁹ coulombs). This positive charge is the defining characteristic of a proton.
- Mass: Approximately 1.673 × 10⁻²⁷ kg. This mass is significantly larger than that of an electron.
- Spin: ½, a quantum property representing intrinsic angular momentum. This spin contributes to the overall magnetic properties of the atom.
- Composition: Protons, unlike electrons, are not fundamental particles. They are composed of three quarks: two up quarks and one down quark, held together by the strong nuclear force.
- Stability: Protons are remarkably stable particles. Free protons (those not bound within an atomic nucleus) have an extremely long lifespan, far exceeding the age of the universe.
The Role of Protons in Atomic Structure: Defining the Element
The number of protons within an atom's nucleus is its atomic number, which uniquely identifies a chemical element. For instance, hydrogen (H) has one proton, helium (He) has two, carbon (C) has six, and so on. This number directly dictates the element's properties and its position on the periodic table. Changing the number of protons fundamentally alters the element itself.
Isotopes: Variations in Neutron Count
While the number of protons determines the element, the number of neutrons can vary within the same element. These variations are called isotopes. Isotopes of the same element have the same number of protons but different numbers of neutrons. This difference in neutron number can affect the atom's stability and mass. Some isotopes are stable, while others are radioactive, undergoing decay to achieve greater stability.
The Strong Nuclear Force: Binding the Nucleus Together
The strong nuclear force is a fundamental force that plays a crucial role in holding protons and neutrons together within the atom's nucleus. This force is incredibly strong at short distances, overcoming the electrostatic repulsion between the positively charged protons. Without the strong nuclear force, the nucleus would instantly disintegrate due to the repulsive forces between protons.
The strength and range of the strong nuclear force are critical parameters in determining the stability of atomic nuclei. Larger nuclei require more neutrons to balance the repulsive forces between protons and maintain stability. This is why heavier elements generally have a higher neutron-to-proton ratio compared to lighter elements.
Protons and Chemical Reactions: The Electron's Dance
Although protons reside within the nucleus and are largely unaffected by chemical reactions, they play an indirect yet crucial role. The number of protons determines the number of electrons an atom possesses in a neutral state. These electrons, located in the atom's outer shells, are the primary participants in chemical bonding.
The positive charge of the protons attracts the negatively charged electrons, maintaining the atom's overall electrical neutrality. When atoms interact, the arrangement and sharing of electrons determine the types of chemical bonds formed (ionic, covalent, metallic), impacting the properties of the resulting molecules and compounds.
Protons in Nuclear Physics and Beyond: Applications and Significance
The study of protons extends far beyond the realm of basic atomic structure. They play critical roles in various scientific fields, including:
Particle Physics: Delving into the Subatomic World
Protons are key players in particle physics research. Experiments involving high-energy collisions of protons, such as those conducted at the Large Hadron Collider (LHC), have provided valuable insights into the fundamental forces of nature and the composition of matter. These experiments help physicists probe the intricacies of the Standard Model of particle physics and search for new particles and phenomena beyond the current understanding.
Nuclear Reactions and Energy Production: Harnessing the Power of the Nucleus
Nuclear reactions involving protons are fundamental to nuclear energy production. Processes like nuclear fission (splitting of heavy atomic nuclei) and nuclear fusion (combining light atomic nuclei) involve changes in the number of protons and neutrons within atomic nuclei, releasing enormous amounts of energy. Understanding proton behavior is crucial to developing safe and efficient nuclear technologies.
Medical Applications: Imaging and Treatment
Protons are used in various medical applications, including proton therapy, a type of radiation therapy that uses beams of protons to target and destroy cancerous tumors. The high precision of proton beams minimizes damage to surrounding healthy tissues, making it a powerful tool in cancer treatment. Proton magnetic resonance imaging (MRI) also utilizes the magnetic properties of protons to create detailed images of the internal structures of the body.
Astrophysics and Cosmology: Unveiling the Universe's Mysteries
The abundance of protons in the universe and their role in stellar nucleosynthesis (the creation of heavier elements within stars) are vital in understanding the universe's evolution. Studying the behavior and distribution of protons provides crucial information regarding the formation of galaxies, stars, and planets.
Conclusion: The Proton's Enduring Importance
The positively charged particle of an atom, the proton, is far more than a simple component of atomic structure. Its properties, interactions, and role in various scientific disciplines highlight its fundamental significance in shaping our understanding of matter, energy, and the universe itself. From the stability of atoms to the workings of stars and the development of advanced technologies, protons continue to fascinate and inspire researchers, pushing the boundaries of human knowledge and unlocking the secrets of the cosmos. Ongoing research in particle physics, nuclear physics, and astrophysics continues to deepen our understanding of this fundamental building block of matter, promising further breakthroughs in the years to come. The journey of exploring the proton and its profound impact on the universe remains an ongoing and endlessly rewarding endeavor.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 4 5 6
Mar 16, 2025
-
What Are Two Elements That Are Liquid At Room Temperature
Mar 16, 2025
-
What Type Of Cell Would Contain More Mitochondria
Mar 16, 2025
-
Which Of The Following Numbers Are Multiples Of 6
Mar 16, 2025
-
The Loudness Of A Persons Voice Depends On The
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about What Is The Positively Charged Particle Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
