What Is The Oxidation State Of Boron In Na2b4o7
Juapaving
May 13, 2025 · 5 min read
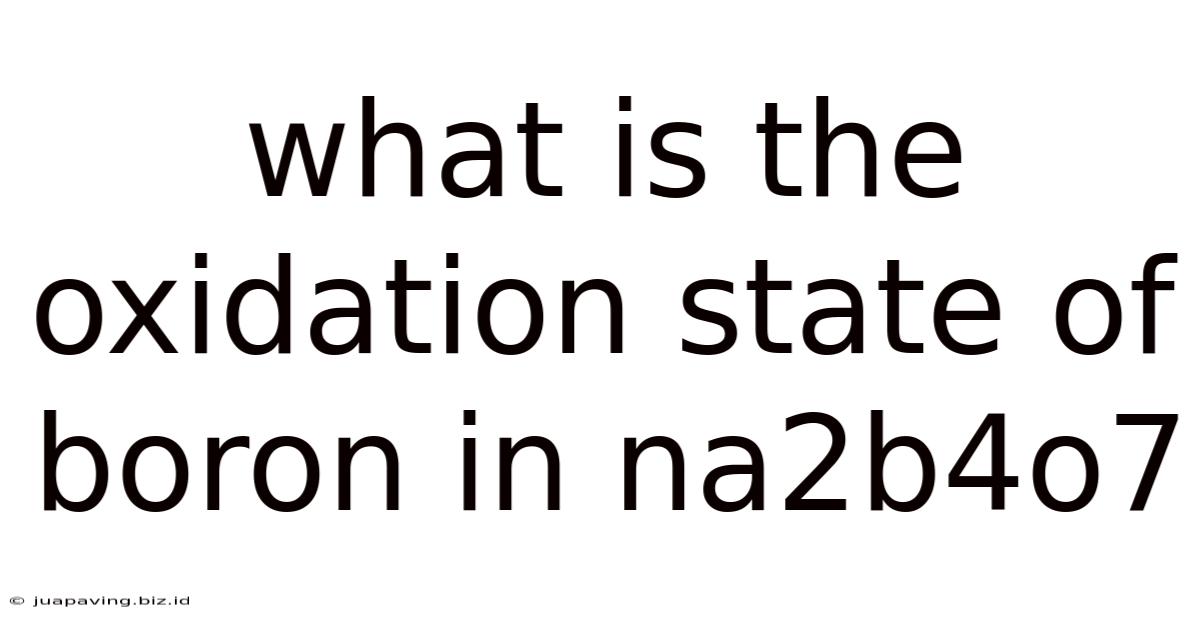
Table of Contents
What is the Oxidation State of Boron in Na₂B₄O₇? A Comprehensive Exploration
Determining the oxidation state of elements within a compound is a fundamental concept in chemistry. This article delves into the specifics of calculating the oxidation state of boron (B) in sodium tetraborate, also known as borax (Na₂B₄O₇). We'll explore the various methods, address potential complexities, and provide a clear, step-by-step explanation suitable for students and enthusiasts alike.
Understanding Oxidation States
Before tackling the specific case of borax, let's briefly review the concept of oxidation states. The oxidation state, also known as oxidation number, is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are completely ionic. This is a useful tool for understanding the electron transfer that occurs during redox reactions. While not a true physical charge, it provides valuable insight into chemical behavior.
Several rules govern the assignment of oxidation states:
- Rule 1: The oxidation state of an atom in its elemental form is always zero. For example, the oxidation state of O₂ is 0, and the oxidation state of Na in metallic sodium is 0.
- Rule 2: The oxidation state of a monatomic ion is equal to its charge. For instance, the oxidation state of Na⁺ is +1, and the oxidation state of Cl⁻ is -1.
- Rule 3: The sum of the oxidation states of all atoms in a neutral molecule is zero.
- Rule 4: The sum of the oxidation states of all atoms in a polyatomic ion equals the charge of the ion.
- Rule 5: In most compounds, the oxidation state of hydrogen is +1 (except in metal hydrides, where it's -1).
- Rule 6: In most compounds, the oxidation state of oxygen is -2 (except in peroxides, where it's -1, and in superoxides, where it's -1/2).
These rules are crucial for systematically determining oxidation states in various compounds.
Calculating the Oxidation State of Boron in Na₂B₄O₇
Now, let's apply these principles to determine the oxidation state of boron in Na₂B₄O₇. This compound, sodium tetraborate, is an important industrial chemical used in various applications, ranging from detergents to glass production. Its structure and bonding are relatively complex, making the oxidation state calculation a bit more involved than simpler compounds.
Step 1: Identify the known oxidation states.
We know the following:
- Sodium (Na) typically has an oxidation state of +1.
- Oxygen (O) typically has an oxidation state of -2.
Step 2: Assign variables.
Let's assign 'x' as the oxidation state of boron (B). Since there are four boron atoms in the formula unit, we'll consider 4x.
Step 3: Apply the rule of zero net charge.
Because Na₂B₄O₇ is a neutral compound, the sum of the oxidation states of all atoms must equal zero. Therefore, we can set up the following equation:
2(+1) + 4x + 7(-2) = 0
Step 4: Solve for x.
Solving this equation for x (the oxidation state of boron):
2 + 4x - 14 = 0 4x = 12 x = +3
Therefore, the oxidation state of boron in Na₂B₄O₇ is +3.
Deeper Dive into the Structure and Bonding of Borax
To further solidify our understanding, let's explore the structure of borax. The formula Na₂B₄O₇ is a simplification; the actual structure is more complex, involving interconnected borate polyanions. Borax contains the tetraborate anion, [B₄O₅(OH)₄]²⁻, which is hydrated in the crystalline structure. The simplified formula Na₂B₄O₇ hides the presence of hydroxide groups.
The boron atoms in the tetraborate anion exhibit a variety of bonding environments. Some boron atoms are bonded to three oxygen atoms, while others are bonded to four. This structural complexity highlights that while the overall average oxidation state of boron is +3, the individual boron atoms may not all have precisely +3. The average oxidation state calculation, however, provides a useful overall representation of the electron distribution.
Common Misconceptions and Potential Pitfalls
It’s crucial to be aware of potential pitfalls when calculating oxidation states. One common misconception is to assume that all atoms of a particular element within a molecule have the same oxidation state. In the case of borax, as discussed above, the bonding environment around each boron atom isn't uniform, leading to slight variations in electron distribution. However, the average oxidation state remains +3.
Another potential pitfall is incorrectly applying the rules of oxidation state assignment. Always carefully consider the exceptions to the general rules, such as the oxidation states of oxygen in peroxides and superoxides. Thorough understanding of these rules is paramount for accurate calculations.
Applications and Importance of Borax
Borax finds widespread applications in various industries and everyday life. Its versatility stems from the unique properties of the borate anion and the ease of its processing.
-
Detergents: Borax is a common ingredient in laundry detergents, acting as a water softener and boosting cleaning efficacy. This is due to its ability to complex with metal ions, which can otherwise interfere with the detergent's effectiveness.
-
Glassmaking: It plays a vital role in the production of glass, acting as a flux – lowering the melting point of silica. This allows for easier processing and production of various glass types.
-
Pesticides: Borax exhibits insecticidal properties and is used in certain pest control applications.
-
Other Applications: The diverse applications of borax also extend to enamel glazes, fertilizers, and fire retardants, among others. Its multifaceted properties are linked to the behavior of the borate anion and the ease of its manipulation in various chemical processes.
Conclusion: The Oxidation State of Boron in Borax Remains Consistent
Despite the structural complexities of borax, the calculated oxidation state of +3 for boron remains consistent and valid. This average oxidation state provides a valuable insight into the overall electron distribution within the compound. This article has demonstrated the step-by-step process of determining the oxidation state, highlighting the importance of understanding the underlying principles and potential complexities. A thorough understanding of oxidation states is fundamental to comprehending various chemical reactions and the properties of compounds like borax. The versatility of borax and its widespread applications further underscore the significance of understanding its chemical makeup. Remember to always carefully consider the rules and exceptions when calculating oxidation states for accurate results.
Latest Posts
Latest Posts
-
Whats The Lcm Of 18 And 24
May 13, 2025
-
Which Of The Following Is A Polynomial
May 13, 2025
-
The Sum Of Potential And Kinetic Energy
May 13, 2025
-
What Is The Molarity Of Water
May 13, 2025
-
Mars Distance From Earth In Light Years
May 13, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidation State Of Boron In Na2b4o7 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.