What Is The Name Of Ca No3 2
Juapaving
Mar 15, 2025 · 5 min read
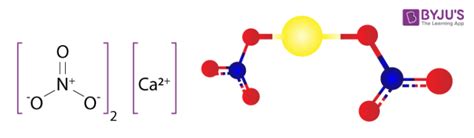
Table of Contents
What is the Name of Ca(NO₃)₂? Unlocking the Secrets of Calcium Nitrate
Calcium nitrate. The name itself might sound a bit intimidating to the uninitiated, but understanding its composition and properties unlocks a world of applications, from agriculture to pyrotechnics. This comprehensive guide delves deep into the fascinating world of Ca(NO₃)₂, exploring its chemical makeup, various names, uses, safety precautions, and environmental impact.
Understanding the Chemical Formula: Ca(NO₃)₂
Let's break down the chemical formula Ca(NO₃)₂. This formula tells us exactly what elements are present and in what ratio:
- Ca: This represents Calcium, an alkaline earth metal crucial for various biological processes.
- (NO₃): This is the nitrate ion, a polyatomic anion composed of one nitrogen atom and three oxygen atoms. The parentheses indicate that there are two nitrate ions associated with each calcium ion.
- ₂: The subscript "2" signifies that there are two nitrate ions (NO₃⁻) for every one calcium ion (Ca²⁺). This ensures the compound has a neutral overall charge.
The balanced ionic charges – +2 from calcium and -1 from each nitrate ion (for a total of -2) – highlight the importance of accurate stoichiometry in chemical formulas.
The Many Names of Ca(NO₃)₂: From Formal to Common
While Ca(NO₃)₂ is the universally accepted chemical formula, the compound boasts several names, depending on the context and audience. These include:
- Calcium Nitrate: This is the most common and widely accepted name. It clearly indicates the constituent elements and is preferred for general use in scientific and educational settings.
- Calcium Dinitrate: This name highlights the presence of two nitrate ions. Although accurate, it's less frequently used than "calcium nitrate."
- Norgessalpeter (Norwegian Saltpeter): This is a historical name referring to the production of calcium nitrate from the Birkeland-Eyde process, an early industrial method of nitrogen fixation.
- Lime Nitrate: This name highlights the presence of the calcium component of the material, useful in agricultural discussions.
Understanding these alternative names is important for navigating different sources of information, especially historical texts and specialized industry literature. However, calcium nitrate remains the most straightforward and widely accepted name.
Applications of Calcium Nitrate: A Versatile Compound
Calcium nitrate finds applications across a variety of fields, leveraging its properties as a source of both calcium and nitrogen:
1. Agriculture: A Boon for Plant Growth
This is perhaps the most significant application of calcium nitrate. It serves as a valuable fertilizer providing two essential plant nutrients:
- Calcium (Ca): Essential for cell wall development, membrane stability, and various enzymatic functions. Calcium deficiency can lead to stunted growth and impaired fruit development.
- Nitrate (NO₃⁻): A crucial source of nitrogen, a primary building block of proteins and nucleic acids. Nitrogen is critical for robust plant growth and yield.
Calcium nitrate's water solubility ensures easy absorption by plants, making it a readily available source of these vital nutrients. Its balanced formulation helps avoid nutrient imbalances, often associated with applying individual calcium and nitrogen sources.
2. Food Industry: A Preservative and More
While less prominent than its agricultural use, calcium nitrate finds limited applications in the food industry. It may be used as:
- A food additive: In specific applications, it can act as a preservative or pH adjuster. Strict regulations control its usage in food products.
- A curing agent: In certain meat products, calcium nitrate may contribute to the curing process.
3. Concrete Industry: Enhancing Strength and Durability
Calcium nitrate is employed in specific concrete applications to enhance its properties. Its addition can influence the hydration process, leading to potential improvements in:
- Strength: Enhanced compressive and tensile strength.
- Durability: Increased resistance to weathering and deterioration.
The use of calcium nitrate in concrete is a specialized niche, often requiring specific expertise and careful control of application procedures.
4. Pyrotechnics: Adding Brilliance to Fireworks
Although less common than agricultural applications, calcium nitrate plays a role in pyrotechnics. It acts as an oxidizer, providing oxygen for combustion and contributing to the vibrant red color in some fireworks displays.
5. Other Uses
The versatility of calcium nitrate extends to other niche applications, including:
- Refrigerant solutions: Calcium nitrate can be incorporated into certain brine solutions used in refrigeration systems.
- Chemical synthesis: It serves as a reagent in specific chemical synthesis reactions.
Safety Precautions and Handling
While calcium nitrate is relatively safe in its solid form, certain precautions are necessary:
- Eye and Skin Protection: Always wear appropriate eye protection and gloves when handling calcium nitrate powder to avoid irritation.
- Avoid Inhalation: Avoid inhaling dust or fumes generated during handling. Good ventilation is essential.
- Storage: Store calcium nitrate in a cool, dry place, away from incompatible materials (strong acids and organic compounds) to prevent potential reactions.
- Fire Hazard: Although not inherently flammable, calcium nitrate can act as an oxidizer, potentially accelerating the combustion of other materials. Keep away from ignition sources.
Always consult the Safety Data Sheet (SDS) for specific handling instructions. The SDS provides comprehensive information on safety procedures, hazard identification, and emergency response protocols.
Environmental Impact of Calcium Nitrate
The environmental impact of calcium nitrate is a complex issue:
- Eutrophication: As a nitrogen-rich fertilizer, excessive use can contribute to eutrophication of water bodies. This process results in algal blooms, oxygen depletion, and harm to aquatic life. Responsible and balanced application is crucial to minimize this impact.
- Acidification: While not as significant as other nitrogen-based fertilizers, improper use can slightly acidify soils.
- Greenhouse Gas Emissions: The production of calcium nitrate can contribute to greenhouse gas emissions, particularly nitrogen oxides. Efficient production methods are important for mitigating this effect.
Sustainable agricultural practices, efficient application methods, and responsible disposal are essential to minimizing the environmental footprint of calcium nitrate usage.
Conclusion: A Multifaceted Compound with Broad Applications
Ca(NO₃)₂, better known as calcium nitrate, is a multifaceted chemical compound with significant implications across diverse sectors. Its crucial role in agriculture, combined with its applications in the food, concrete, and pyrotechnics industries, underscores its versatility. Understanding its properties, safe handling procedures, and potential environmental impact is essential for its responsible and beneficial utilization. This knowledge helps ensure its continued contribution to various industries while minimizing any negative environmental effects. From its historical name, Norgessalpeter, to its modern applications, calcium nitrate remains a fascinating and important chemical compound.
Latest Posts
Latest Posts
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
-
How Do You Find The Inverse Of A Relation
Mar 17, 2025
-
Does Cold Air Go Up Or Down
Mar 17, 2025
-
Least Common Multiple Of 20 And 3
Mar 17, 2025
-
Function Of The Motor End Plate
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Name Of Ca No3 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
