What Is The Name For H2so4
Juapaving
Mar 16, 2025 · 6 min read
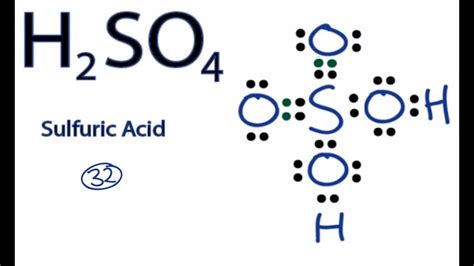
Table of Contents
What is the Name for H₂SO₄? A Deep Dive into Sulfuric Acid
Sulfuric acid, represented by the chemical formula H₂SO₄, is a ubiquitous and incredibly important chemical compound. Its name, however, might seem deceptively simple, concealing the rich history and diverse applications of this powerful substance. This comprehensive guide will delve into the nomenclature of H₂SO₄, exploring its various names, its chemical properties, and its widespread use across numerous industries.
Understanding the Nomenclature of H₂SO₄
The most common and widely accepted name for H₂SO₄ is sulfuric acid. This name clearly indicates the compound's composition: it's an acid derived from sulfur. But the story doesn't end there. Let's unpack the different ways this vital chemical is referred to:
1. Sulfuric Acid: The Standard Name
Sulfuric acid is the IUPAC (International Union of Pure and Applied Chemistry) name, the globally recognized standard for chemical nomenclature. This systematic name reflects the compound's structure and composition, providing clarity and consistency in scientific communication. The "sulfur" part denotes the presence of sulfur as the central atom, and "ic" indicates the highest oxidation state of sulfur (+6) in the compound. The "acid" suffix clarifies its acidic nature, meaning it donates protons (H⁺ ions) in aqueous solutions.
2. Oil of Vitriol: A Historical Name
Before the establishment of modern chemical nomenclature, sulfuric acid was known as oil of vitriol. This evocative name stems from its early preparation methods. Alchemists and early chemists produced sulfuric acid by heating green vitriol (ferrous sulfate heptahydrate, FeSO₄·7H₂O), a naturally occurring mineral, leading to a viscous, oily liquid. The term "vitriol" itself is derived from the Latin word "vitreus," meaning "glassy," reflecting the crystalline appearance of certain metal sulfates. While less frequently used today, "oil of vitriol" provides a glimpse into the rich history of this essential chemical.
3. Dihydrogen Sulfate: A Systematic Alternative
Using the systematic naming conventions, another acceptable name for H₂SO₄ is dihydrogen sulfate. This nomenclature emphasizes the presence of two hydrogen atoms directly bonded to the sulfate anion (SO₄²⁻). While less commonly used than "sulfuric acid" in everyday contexts, this name is useful in certain chemical discussions, particularly when emphasizing the bonding structure of the molecule.
4. Hydrogen Sulfate (Bisulfate): The Anion's Perspective
When sulfuric acid loses one proton (H⁺), it forms the hydrogen sulfate or bisulfate anion (HSO₄⁻). While not a name for the acid itself, understanding this anionic form is critical for comprehending the acid's behavior in reactions and solutions. Salts containing the bisulfate anion are commonly encountered in various applications.
Properties of Sulfuric Acid: Why is it so Important?
The widespread use of H₂SO₄ is directly linked to its unique chemical properties:
1. Strong Acidity: The Proton Donor
Sulfuric acid is a strong acid, meaning it readily donates protons (H⁺ ions) when dissolved in water. This high acidity is responsible for its corrosive nature and its effectiveness in many chemical processes. Its strong acidic nature makes it an excellent catalyst in many industrial reactions.
2. Dehydrating Agent: Removing Water Molecules
Sulfuric acid's exceptional ability to absorb water makes it a potent dehydrating agent. This property is utilized in various applications, including drying gases and desiccating substances. The strong affinity for water allows the acid to remove water molecules from organic compounds, sometimes leading to charring or carbonization.
3. Oxidizing Agent: Electron Acceptor
Concentrated sulfuric acid can act as a powerful oxidizing agent, accepting electrons from other substances. This property is particularly important in certain industrial processes and in some chemical reactions. The oxidation potential of sulfuric acid can lead to interesting and sometimes hazardous reactions.
4. High Boiling Point: Stability at High Temperatures
Sulfuric acid boasts a relatively high boiling point (337 °C), making it suitable for use in high-temperature processes. This stability at elevated temperatures enhances its usefulness as a catalyst and reaction medium in numerous industrial settings.
Applications of Sulfuric Acid: A Multifaceted Chemical
The remarkable properties of sulfuric acid translate into its vast applications across various industries:
1. Fertilizer Production: Nourishing the Crops
Sulfuric acid is a crucial component in the production of phosphatic fertilizers. It reacts with phosphate rock to produce phosphoric acid, which is then used to create various phosphate fertilizers essential for agriculture. The global demand for fertilizers significantly drives the production of sulfuric acid.
2. Chemical Synthesis: The Building Block
Sulfuric acid serves as a catalyst and reagent in countless chemical synthesis reactions. Its acidic properties and dehydrating capabilities are utilized in the production of a vast array of chemicals, including dyes, pigments, detergents, and pharmaceuticals.
3. Metal Processing: Refining and Cleaning
Sulfuric acid plays a vital role in metal processing and refining. It is used in the cleaning, pickling, and etching of metals, removing impurities and preparing surfaces for further processing. This is crucial in the production of various metal components and products.
4. Petroleum Refining: Improving Fuels
Sulfuric acid is employed in petroleum refining to improve the quality of fuels and lubricants. It is used in alkylation processes, which enhance the octane rating of gasoline, and in various refining operations.
5. Battery Production: Powering Devices
Sulfuric acid is the electrolyte in lead-acid batteries, which are widely used in automobiles, backup power systems, and other applications. Its ability to conduct electricity and participate in electrochemical reactions makes it an indispensable component of these energy storage devices.
6. Textile Industry: Treating Fabrics
In the textile industry, sulfuric acid is utilized in various processes, including the treatment of fabrics, the production of certain dyes, and the processing of fibers. Its role in these processes contributes to the quality and properties of the final textile products.
7. Other Applications: A Diverse Range
The versatility of sulfuric acid extends beyond these major applications. It finds use in numerous other sectors, including:
- Paper production: Processing wood pulp and whitening paper.
- Food processing: (though with careful regulation due to its corrosive nature) Some limited applications exist in specialized food processing, requiring strict safety protocols.
- Water treatment: Used in some specialized water treatment processes, although other chemicals are generally preferred for large scale water treatment.
- Wastewater treatment: Specific applications in treating industrial wastewater.
Safety Precautions: Handling with Care
Due to its strong corrosive nature and potential for severe chemical burns, handling sulfuric acid requires meticulous safety precautions. Appropriate personal protective equipment (PPE), including gloves, goggles, and lab coats, is essential. Proper ventilation is also crucial to avoid inhaling the acid fumes. Accidental spills must be handled with extreme caution, and appropriate neutralization procedures should be followed.
Conclusion: The Unsung Hero of Chemistry
H₂SO₄, commonly known as sulfuric acid, is much more than just a chemical formula. It is a testament to the power and versatility of chemical compounds, playing a critical role in numerous industries and impacting our daily lives in countless ways. Its historical names like "oil of vitriol" reveal a rich legacy, while its modern systematic names highlight its precise chemical composition. Understanding its properties and applications is crucial for appreciating its importance in the modern world and handling it safely. From fertilizer production to battery technology, sulfuric acid remains a cornerstone of modern chemistry and industrial processes. Remember to always prioritize safety when handling this powerful chemical.
Latest Posts
Latest Posts
-
How Do You Find The Inverse Of A Relation
Mar 17, 2025
-
Does Cold Air Go Up Or Down
Mar 17, 2025
-
Least Common Multiple Of 20 And 3
Mar 17, 2025
-
Function Of The Motor End Plate
Mar 17, 2025
-
A Push Or A Pull Is Called
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Name For H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
