What Is The Molar Mass Of Kclo3
Juapaving
Mar 18, 2025 · 5 min read
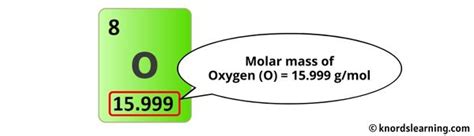
Table of Contents
What is the Molar Mass of KClO3? A Deep Dive into Potassium Chlorate
Potassium chlorate (KClO3) is a chemical compound with a wide array of applications, from its use in matches and fireworks to its role in various chemical experiments and industrial processes. Understanding its properties, particularly its molar mass, is crucial for accurate stoichiometric calculations and safe handling. This comprehensive guide will delve into the calculation of KClO3's molar mass, explore its significance, and discuss some of its key applications.
Understanding Molar Mass
Before we calculate the molar mass of KClO3, let's establish a fundamental understanding of the concept. Molar mass refers to the mass of one mole of a substance. A mole, represented by the symbol "mol," is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of elementary entities (atoms, molecules, ions, etc.). The molar mass is numerically equivalent to the atomic mass (for elements) or molecular mass (for compounds) expressed in grams per mole (g/mol).
Calculating the Molar Mass of KClO3
To calculate the molar mass of KClO3, we need to consider the atomic masses of its constituent elements: potassium (K), chlorine (Cl), and oxygen (O). These atomic masses are typically found on the periodic table.
- Potassium (K): Approximately 39.10 g/mol
- Chlorine (Cl): Approximately 35.45 g/mol
- Oxygen (O): Approximately 16.00 g/mol
KClO3 contains:
- 1 potassium atom (K)
- 1 chlorine atom (Cl)
- 3 oxygen atoms (O)
Therefore, the molar mass of KClO3 is calculated as follows:
(1 x atomic mass of K) + (1 x atomic mass of Cl) + (3 x atomic mass of O)
= (1 × 39.10 g/mol) + (1 × 35.45 g/mol) + (3 × 16.00 g/mol)
= 39.10 g/mol + 35.45 g/mol + 48.00 g/mol
= 122.55 g/mol
Therefore, the molar mass of KClO3 is approximately 122.55 grams per mole.
Significance of Molar Mass in Chemical Calculations
The molar mass of KClO3 plays a vital role in various chemical calculations, including:
1. Stoichiometric Calculations:
Understanding the molar mass allows for accurate stoichiometric calculations. This means determining the quantitative relationships between reactants and products in a chemical reaction. For example, if you want to determine how much KClO3 is needed to produce a specific amount of oxygen gas in a decomposition reaction, the molar mass is essential for converting between grams and moles.
2. Concentration Calculations:
Molar mass is crucial for preparing solutions of known concentrations. For instance, if you need to prepare a 1 M (molar) solution of KClO3, you would use the molar mass to calculate the exact mass of KClO3 needed to dissolve in a specific volume of solvent.
3. Determining Empirical and Molecular Formulas:
Molar mass is a key factor in determining the empirical and molecular formulas of compounds. The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula represents the actual number of atoms in a molecule. Knowing the molar mass helps distinguish between these formulas.
4. Gas Law Calculations:
In situations involving gases, the molar mass is used in conjunction with the ideal gas law (PV = nRT) to calculate properties like volume, pressure, or temperature. This is particularly relevant in reactions involving gaseous products or reactants.
Applications of Potassium Chlorate (KClO3)
Potassium chlorate finds diverse applications due to its strong oxidizing properties:
1. Matches and Fireworks:
KClO3 is a common oxidizer in matches and fireworks, providing the oxygen necessary for rapid combustion. Its presence enhances the burning intensity and brilliance of the flame.
2. Chemical Experiments:
In various chemical experiments, KClO3 serves as a convenient source of oxygen. Its thermal decomposition releases oxygen gas, useful for demonstrating the properties of oxygen or for generating oxygen in a controlled environment. It's crucial to handle KClO3 with extreme caution during experiments, as it can be explosive under certain conditions.
3. Bleaching Agent:
KClO3 can act as a bleaching agent in specific industrial processes, oxidizing colored substances and removing impurities.
4. Weed Killer:
Due to its oxidizing properties, potassium chlorate has found limited use as a herbicide (weed killer) in the past, though its use is now less common due to environmental concerns and the availability of less hazardous alternatives.
5. Other Industrial Applications:
Other applications of potassium chlorate include its use in textile manufacturing, metal processing, and as a component in certain explosives.
Safety Precautions when Handling KClO3
It is absolutely crucial to emphasize the safety precautions associated with handling potassium chlorate:
- Avoid contact with reducing agents: KClO3 is a strong oxidizing agent and can react violently with reducing agents, potentially leading to explosions or fires. Keep it away from combustible materials.
- Avoid friction and shock: Friction or impact can trigger the decomposition of KClO3, potentially causing explosions. Handle it gently and avoid any activities that could generate friction or shock.
- Proper storage: Store KClO3 in a cool, dry, well-ventilated area, away from incompatible materials.
- Protective equipment: Always wear appropriate personal protective equipment (PPE), including gloves, safety glasses, and a lab coat, when handling KClO3.
- Never heat KClO3 in a confined space: Heating KClO3 can lead to rapid decomposition and the release of oxygen gas. Never perform such heating in a sealed container or confined area.
Conclusion
The molar mass of KClO3, approximately 122.55 g/mol, is a fundamental property essential for various chemical calculations and applications. Understanding this value is crucial for accurate stoichiometric calculations, solution preparation, and the safe and effective use of potassium chlorate in various fields. However, it's paramount to remember that KClO3 is a powerful oxidizing agent and should be handled with extreme care, adhering strictly to safety protocols to prevent accidents. This knowledge equips chemists, students, and anyone working with this compound with the necessary information for its safe and responsible use. Always prioritize safety when working with chemicals.
Latest Posts
Latest Posts
-
Which Is Bigger Pounds Or Kilograms
Mar 18, 2025
-
What Is The Lcm Of 24 And 12
Mar 18, 2025
-
Neutral Atoms Contain Equal Numbers Of
Mar 18, 2025
-
Which Is More Important Brain Or Heart
Mar 18, 2025
-
How Many Even Primes Are There
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Kclo3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
