What Is The Freezing Point Of Water In Degrees Kelvin
Juapaving
Mar 29, 2025 · 5 min read
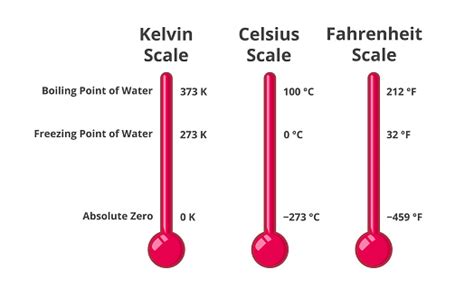
Table of Contents
What is the Freezing Point of Water in Degrees Kelvin?
The freezing point of water, a seemingly simple concept, is crucial to numerous scientific fields and everyday life. While we often talk about water freezing at 0 degrees Celsius or 32 degrees Fahrenheit, understanding its freezing point in Kelvin requires delving into the fundamentals of the Kelvin scale and its relationship to other temperature scales. This article will explore the freezing point of water in degrees Kelvin, discussing the underlying principles, its significance in various contexts, and some common misconceptions.
Understanding the Kelvin Scale
The Kelvin scale, also known as the absolute temperature scale, is a thermodynamic temperature scale where zero represents absolute zero – the theoretical absence of all thermal energy. Unlike Celsius and Fahrenheit, which use arbitrary reference points (like the freezing and boiling points of water), the Kelvin scale is based on fundamental physical principles. This makes it a preferred scale for scientific applications.
Key features of the Kelvin scale:
- Absolute Zero: 0 Kelvin (0 K) represents the lowest possible temperature, where all molecular motion ceases. It's an unattainable point in practice, but a crucial theoretical concept.
- No Negative Values: The Kelvin scale only uses positive values, eliminating the possibility of negative temperatures. This simplifies many thermodynamic calculations.
- Relationship to Celsius: The Kelvin scale is directly related to the Celsius scale. The conversion is straightforward: K = °C + 273.15. This means that a temperature difference of 1 Kelvin is equal to a temperature difference of 1 degree Celsius.
Deriving the Freezing Point of Water in Kelvin
Now, let's apply our understanding of the Kelvin scale to determine the freezing point of water. We know that water freezes at 0 degrees Celsius (°C). Using the conversion formula:
K = °C + 273.15
We can calculate the freezing point in Kelvin:
K = 0°C + 273.15 = 273.15 K
Therefore, the freezing point of water is 273.15 Kelvin.
This value is fundamental in various scientific calculations and applications, forming the basis for many thermodynamic equations and experiments.
The Significance of 273.15 K in Science and Engineering
The precise value of 273.15 K for the freezing point of water is not just a number; it has profound implications across various scientific and engineering disciplines. Let's explore some key applications:
1. Thermodynamics and Physical Chemistry
Many fundamental equations in thermodynamics, such as the ideal gas law and various phase transition calculations, rely on absolute temperature (Kelvin). Using Kelvin ensures accurate predictions and modeling of systems involving temperature changes and phase transitions like freezing and melting.
2. Material Science and Engineering
Understanding the behavior of materials at various temperatures, including their melting and freezing points, is critical in material science and engineering. Expressing temperatures in Kelvin provides a consistent and accurate framework for analyzing and predicting material properties at different temperature regimes. This is especially crucial in designing materials for extreme environments or applications involving phase transitions.
3. Cryogenics and Low-Temperature Physics
Cryogenics deals with the production and application of very low temperatures. The Kelvin scale is essential in this field, allowing for precise temperature control and measurement in experiments involving superconductivity, superfluidity, and other low-temperature phenomena. The freezing point of water at 273.15 K provides a crucial reference point for calibrating cryogenic equipment and understanding the behavior of materials at extremely low temperatures.
4. Meteorology and Climate Science
While Celsius is commonly used in weather reports, the Kelvin scale plays a vital role in climate modeling and atmospheric science. Many atmospheric processes, such as cloud formation and precipitation, are sensitive to temperature changes, making the use of the absolute temperature scale critical for accurate modeling and forecasting. Understanding the freezing point of water in Kelvin is crucial for simulating phase transitions in the atmosphere, impacting weather patterns and climate projections.
5. Astrophysics and Cosmology
The Kelvin scale is extensively used in astrophysics and cosmology to measure the temperatures of stars, galaxies, and other celestial bodies. Understanding the behavior of matter at extreme temperatures, expressed in Kelvin, is fundamental to understanding the processes governing the universe. The freezing point of water, though seemingly mundane in this context, still provides a fundamental reference point for understanding the broader range of temperatures found in the cosmos.
Common Misconceptions about the Freezing Point of Water
While the freezing point of water in Kelvin (273.15 K) is well-established, certain misconceptions can arise.
1. The Confusion between 273 K and 273.15 K
Sometimes, the freezing point of water is approximated as 273 K. While this is a close approximation, the more precise value of 273.15 K is crucial for high-precision scientific work and calculations. The 0.15 K difference might seem insignificant, but in certain applications, especially those dealing with small temperature variations, it can lead to noticeable errors.
2. Assuming the Freezing Point is Always 273.15 K
It's important to remember that the freezing point of water at 273.15 K is under standard pressure conditions (1 atmosphere). Changes in pressure can affect the freezing point slightly. At higher pressures, the freezing point can be slightly lower, while at lower pressures, it can be slightly higher. This is a consequence of the phase diagram of water, which exhibits unusual behavior compared to most other substances.
3. Ignoring the Significance of the Absolute Scale
Using Celsius or Fahrenheit for calculations involving thermodynamics or other temperature-dependent processes can lead to inaccurate results. The Kelvin scale's absolute nature ensures that calculations are consistent and reliable, particularly those involving ratios of temperatures or energy changes.
Conclusion: The Importance of Precision and Understanding
The freezing point of water at 273.15 Kelvin is a fundamental constant in science and engineering. Its significance extends far beyond simple observations of ice formation. Precisely understanding and applying this value is crucial for accurate calculations, reliable predictions, and a deeper understanding of the physical world around us, from microscopic processes to the vast expanse of the cosmos. By appreciating the foundational principles of the Kelvin scale and the specific value of 273.15 K for the freezing point of water, we gain a more profound appreciation for the interconnectedness of scientific concepts and their practical applications in our daily lives. Remember, the seemingly simple observation of freezing water holds a wealth of scientific significance, emphasizing the power of precise measurement and the importance of understanding the underlying physical principles.
Latest Posts
Latest Posts
-
Is Oxygen A Metal Nonmetal Or Metalloid
Mar 31, 2025
-
Keyboard Is A Hardware Or Software
Mar 31, 2025
-
Is 45 A Multiple Of 9
Mar 31, 2025
-
Forward Primer And Reverse Primer In Pcr
Mar 31, 2025
-
Words That Begin With A H
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Freezing Point Of Water In Degrees Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
