What Is The Freezing Point For Fahrenheit
Juapaving
Mar 29, 2025 · 6 min read
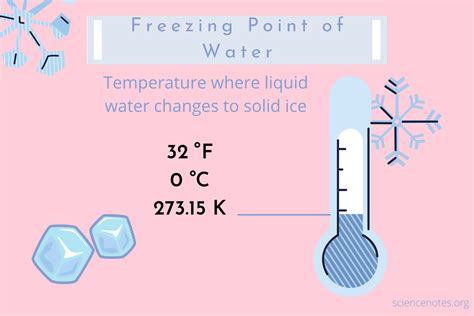
Table of Contents
What is the Freezing Point for Fahrenheit? Understanding Temperature Scales and Their Applications
The freezing point of water is a fundamental concept in understanding temperature and its impact on various aspects of our lives, from everyday activities to scientific research. While the Celsius scale sets the freezing point of water at 0°C, the Fahrenheit scale, commonly used in the United States, places it at 32°F. This seemingly simple difference leads to much confusion and a need for a deeper understanding of the two scales and their historical development. This article delves into the intricacies of the Fahrenheit scale, exploring its origins, its relationship with other temperature scales, and its widespread—though diminishing—application.
The History and Development of the Fahrenheit Scale
The Fahrenheit scale, named after its inventor, Daniel Gabriel Fahrenheit, a German physicist, emerged in the early 18th century. Unlike the Celsius scale, which is based on the freezing and boiling points of water, Fahrenheit’s scale was originally defined using a different set of reference points.
Original Reference Points:
Fahrenheit’s initial calibration used three fixed points:
-
Zero degrees (°F): This was defined as the temperature of an ice-salt mixture (a solution of ice and ammonium chloride). This mixture produced a lower temperature than pure freezing water and provided a convenient lower limit for the scale. This was a crucial point for Fahrenheit, as it allowed for measurements below the freezing point of water, which was a significant advancement at the time.
-
32 degrees (°F): This was established as the freezing point of pure water.
-
96 degrees (°F): This was initially designated as the approximate temperature of the human body.
Evolution of the Scale:
Over time, the definition of the human body temperature reference point was refined and adjusted, leading to minor modifications in the scale's calibration. However, the freezing and boiling points of water remained integral to the scale's definition, with the boiling point fixed at 212°F.
Understanding the Relationship Between Fahrenheit and Celsius
The Fahrenheit and Celsius scales are related through a simple mathematical conversion formula. Understanding this relationship is crucial for anyone needing to convert between the two. The conversion formula is:
°C = (°F - 32) × 5/9
Conversely, to convert Celsius to Fahrenheit:
°F = (°C × 9/5) + 32
Why the Conversion Formula?
The differing scales arise from their different origins and reference points. The factor 5/9 or 9/5 accounts for the different intervals between the freezing and boiling points of water on each scale. The subtraction or addition of 32 is needed to adjust for the different starting points (0°C versus 32°F).
The Significance of 32°F: The Freezing Point of Water
The freezing point of water at 32°F is a crucial benchmark in numerous fields:
-
Everyday Life: This temperature is vital for everyday weather forecasting, determining if water will freeze in pipes, understanding conditions for outdoor activities, and many other applications.
-
Agriculture: Farmers rely on accurate temperature readings in Fahrenheit to manage crops effectively, preventing frost damage and optimizing irrigation. Knowledge of freezing temperatures is crucial for planting and harvesting schedules.
-
Science and Engineering: 32°F serves as a constant in various scientific experiments and engineering calculations, often involving materials' behaviors at low temperatures, especially in studies relating to phase transitions.
-
Food Safety: Maintaining food below 32°F (or 0°C) is critical for food safety, preventing bacterial growth and ensuring food's shelf life. This is paramount in food processing and storage.
-
Construction and Infrastructure: Construction professionals need to consider freezing temperatures when designing structures and infrastructure to prevent damage from ice and frost. This is especially important in regions experiencing harsh winters.
The Freezing Point and Phase Transitions
The freezing point of water at 32°F marks the transition from the liquid to the solid phase. At this temperature, under normal atmospheric pressure, water molecules lose their kinetic energy and form a crystalline structure, converting into ice. This phase transition is reversible, with ice melting back into liquid water at the same temperature.
Factors Affecting the Freezing Point
While 32°F is the standard freezing point for pure water at sea level, several factors can influence this temperature:
-
Pressure: Increased pressure slightly lowers the freezing point of water, a phenomenon important in high-altitude situations or deep-sea environments.
-
Impurities: Dissolved substances in water, such as salts or other solutes, can lower the freezing point. This phenomenon is commonly used in de-icing road surfaces, where salt is added to lower the freezing point of water and prevent ice formation.
-
Supercooling: Under certain conditions, water can be cooled below 32°F without freezing. This supercooled water is in a metastable state and can quickly freeze if disturbed or subjected to nucleation.
Applications of Understanding the Freezing Point
Understanding the freezing point of water in Fahrenheit has countless practical applications, some of which have already been mentioned. Here are a few more examples showcasing the practical importance of this knowledge:
-
Hydration of Concrete: Concrete hydration processes are greatly affected by temperature, with freezing temperatures leading to damage. Contractors consider freezing temperatures carefully to schedule work and manage materials.
-
Cryopreservation: Freezing tissues and cells at precise temperatures below 32°F is crucial in cryopreservation, a technique used to store biological materials for extended periods. Precise temperature control is vital to ensure cell viability.
-
Winter Sports: Winter sports such as skiing, snowboarding, and ice skating rely heavily on the freezing point of water for creating ideal conditions. Temperature monitoring is crucial for maintaining the proper snow and ice surfaces.
The Fahrenheit Scale's Future
While the Celsius scale is increasingly becoming the globally preferred standard for scientific and meteorological applications, the Fahrenheit scale retains its significance in the United States. However, given the global push towards standardization and the advantages of the Celsius scale's simpler metric-based system, a gradual shift towards Celsius is expected, particularly in educational settings and scientific publications. Understanding both systems, however, remains essential for navigating the various contexts in which each scale is used.
Conclusion: The Enduring Relevance of 32°F
In conclusion, the freezing point of water at 32°F is a significant landmark in the Fahrenheit scale and holds practical importance across numerous disciplines. While the Celsius scale gains increasing global traction, the Fahrenheit scale continues to be relevant, especially in the United States. Understanding the conversion between these scales and the factors affecting the freezing point remains critical for effective decision-making in numerous fields, from everyday life to cutting-edge scientific research. The knowledge of 32°F as the freezing point of water isn't merely an academic detail; it’s a fundamental piece of information impacting our daily lives and the progress of science and technology.
Latest Posts
Latest Posts
-
Dna Is A Polymer Made From What Monomer Units
Mar 31, 2025
-
What Are All The Multiples Of 8
Mar 31, 2025
-
Is Wax Melting A Physical Change
Mar 31, 2025
-
The Gcf Of 12 And 18
Mar 31, 2025
-
Find Area Between Two Curves Calculator
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Freezing Point For Fahrenheit . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
