Is Wax Melting A Physical Change
Juapaving
Mar 31, 2025 · 5 min read
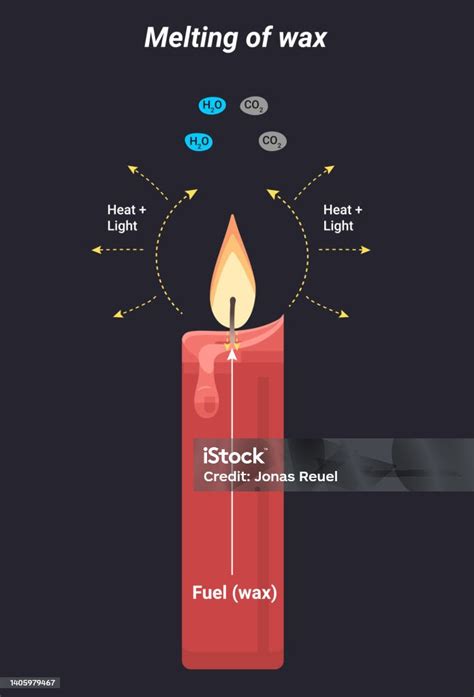
Table of Contents
Is Wax Melting a Physical Change? A Deep Dive into Phase Transitions
The question of whether melting wax constitutes a physical or chemical change is a common one, particularly in the context of science education. While seemingly straightforward, the answer requires a nuanced understanding of the fundamental differences between physical and chemical changes and the specific properties of wax. This article will delve deep into this topic, examining the process of wax melting, the characteristics of physical changes, and the evidence supporting the classification of wax melting as a physical change. We'll also explore related concepts to provide a complete and comprehensive understanding.
Understanding Physical and Chemical Changes
Before we tackle the wax melting conundrum, let's establish a clear definition of physical and chemical changes. This distinction is crucial for correctly classifying any transformation matter undergoes.
Physical changes are alterations that affect the form or appearance of a substance but do not alter its chemical composition. These changes are often reversible. Examples include changes in state (melting, freezing, boiling, condensation, sublimation), dissolving, and changes in shape or size. The substance remains fundamentally the same; only its physical properties have changed.
Chemical changes, also known as chemical reactions, involve the rearrangement of atoms to form new substances with different chemical properties. These changes are often irreversible and are accompanied by observable changes like color change, gas production, temperature change, or precipitation. The original substance is transformed into something fundamentally different.
Analyzing the Melting of Wax: A Physical Transformation
Wax, typically a mixture of hydrocarbons, undergoes a phase transition when heated. This transition is from a solid state to a liquid state – a process we call melting. Let's examine this process in detail to determine its nature:
1. No New Substance is Formed:
The most significant indicator of a physical change is the absence of new substances. When wax melts, the individual hydrocarbon molecules that constitute the wax remain intact. They simply gain kinetic energy, overcoming the intermolecular forces holding them in a rigid structure, and thus transition to a more mobile, liquid state. There's no breaking or formation of chemical bonds; the chemical composition of the wax remains unchanged.
2. Reversibility:
The melting of wax is a reversible process. Upon cooling, the liquid wax solidifies, returning to its original solid form. This reversibility is a hallmark of physical changes. The ability to regain the original state underscores that no fundamental chemical alteration has occurred.
3. Change in Physical Properties:
Melting wax showcases a clear change in its physical properties. The solid wax is rigid, maintains its shape, and possesses a relatively low fluidity. The melted wax, on the other hand, is fluid, takes the shape of its container, and exhibits a high fluidity. These changes in state, shape, and fluidity are consistent with physical transformations.
4. No Energy Release Associated with Bond Formation or Breaking:
Chemical changes are often accompanied by energy changes, typically manifested as heat release (exothermic) or absorption (endothermic). While melting wax does require energy input (it's endothermic), this energy is used to overcome intermolecular forces, not to break chemical bonds within the wax molecules. The energy is used for a phase change, not a chemical reaction.
5. Microscopic Examination:
At a microscopic level, the molecules in solid wax are arranged in a structured, ordered pattern. Melting disrupts this order, resulting in a more random arrangement of molecules in the liquid state. However, the molecules themselves remain unchanged; their chemical structure is preserved.
Types of Wax and their Melting Behavior
While the above discussion focuses on the general melting behavior of wax, it's important to acknowledge that different types of waxes exist, each with its own unique composition and melting point. Paraffin wax, beeswax, and soy wax are just a few examples. However, regardless of the specific type of wax, the melting process remains fundamentally a physical change. The differences lie in the melting point and the specific hydrocarbon composition, but the underlying mechanism – the transition from a solid to a liquid state without altering the chemical structure – remains the same.
Common Misconceptions about Wax Melting
Despite the clear evidence supporting the classification of wax melting as a physical change, some misconceptions persist:
- The appearance of smoke or odor: Some waxes, especially those containing additives or impurities, may produce a slight odor or a small amount of smoke upon melting. However, this does not signify a chemical change. The smoke and odor usually result from the vaporization of volatile components or the decomposition of impurities, not a fundamental alteration of the wax's chemical structure.
- Color change: A slight color change can also occur during the melting of some waxes. This change is often due to the rearrangement of wax molecules or the distribution of pigments, not a chemical reaction.
- Irreversible Changes: While melting wax is reversible, some irreversible changes might occur if the wax is subjected to extremely high temperatures or prolonged heating. This could lead to thermal decomposition – a chemical change. But this is not the typical scenario when wax is simply melted and cooled.
Differentiating Wax Melting from Chemical Changes
To further solidify the understanding that wax melting is a physical change, let's compare it to a clear example of a chemical change: burning wax.
While melting wax involves a change of state, burning wax is a combustion reaction. This process involves the reaction of wax with oxygen, breaking the carbon-carbon and carbon-hydrogen bonds to produce carbon dioxide, water, and heat. The original wax molecules are fundamentally altered; new substances are formed. This is a clear example of a chemical change. The key difference lies in the involvement of oxygen and the breaking and forming of chemical bonds.
Conclusion: Wax Melting Remains a Physical Phenomenon
In conclusion, overwhelming evidence supports the categorization of wax melting as a physical change. The process involves a transition of state, driven by changes in kinetic energy and intermolecular forces, without altering the fundamental chemical composition of the wax. The reversibility, absence of new substances, and the preservation of the chemical structure firmly place wax melting within the realm of physical phenomena. Understanding this distinction is crucial for a deeper understanding of matter and its transformations. The seemingly simple act of melting wax provides a valuable lesson in the fundamental principles of chemistry and physics.
Latest Posts
Latest Posts
-
Which Group Of Plants Lack True Leaves And Roots
Apr 02, 2025
-
How Are The Processes Of Photosynthesis And Cellular Respiration Related
Apr 02, 2025
-
What Is The Relationship Between Length And Resistance
Apr 02, 2025
-
How Are A Square And Rhombus Alike
Apr 02, 2025
-
How Many Factors Does 11 Have
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Wax Melting A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
