Is Salt A Compound Mixture Or Element
Juapaving
Mar 18, 2025 · 5 min read
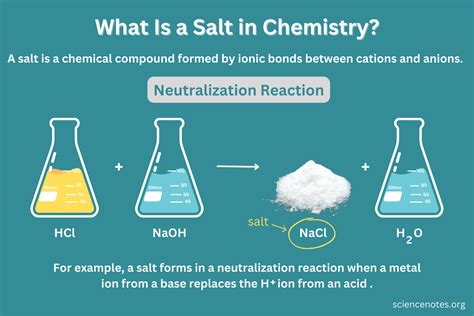
Table of Contents
Is Salt a Compound, Mixture, or Element? A Deep Dive into Chemical Classification
Salt, a ubiquitous substance in our kitchens and beyond, often sparks curiosity about its fundamental nature. Is it an element, a compound, or a mixture? Understanding this requires delving into the core concepts of chemistry. This comprehensive guide will not only answer this question definitively but also explore the broader implications of chemical classification.
Understanding the Basics: Elements, Compounds, and Mixtures
Before we classify salt, let's establish a firm understanding of the three fundamental categories of matter:
Elements:
Elements are the simplest form of matter. They are pure substances consisting of only one type of atom. These atoms cannot be broken down into simpler substances through chemical reactions. Examples include oxygen (O), hydrogen (H), carbon (C), and gold (Au). The periodic table organizes and displays all known elements.
Compounds:
Compounds are pure substances formed when two or more different elements chemically combine in a fixed ratio. This combination involves the formation of chemical bonds, which are strong forces holding the atoms together. The properties of a compound are distinctly different from the properties of its constituent elements. For example, water (H₂O) is a compound formed from hydrogen and oxygen. Water's properties are vastly different from those of hydrogen gas and oxygen gas. The ratio of hydrogen to oxygen in water is always 2:1.
Mixtures:
Mixtures are combinations of two or more substances that are not chemically bonded. The substances in a mixture retain their individual properties, and the ratio of the components can vary. Mixtures can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water).
The Case of Salt: A Chemical Compound
Now, let's focus on salt, specifically table salt, which is chemically known as sodium chloride (NaCl). Salt is definitively a compound, not an element or a mixture. Here's why:
-
Fixed Ratio: Sodium chloride always consists of sodium (Na) and chlorine (Cl) atoms in a 1:1 ratio. This fixed ratio is crucial for identifying it as a compound. Any deviation from this ratio would result in a different substance.
-
Chemical Bonds: Sodium and chlorine atoms are held together by strong ionic bonds. Ionic bonds form when one atom (sodium) donates an electron to another atom (chlorine), creating oppositely charged ions that attract each other. This electrostatic attraction creates a stable crystal lattice structure characteristic of salt.
-
Distinct Properties: The properties of sodium chloride (salt) are vastly different from the properties of its constituent elements, sodium and chlorine. Sodium is a highly reactive metal, while chlorine is a toxic gas. Salt, however, is a crystalline solid that is relatively unreactive and crucial for various biological processes.
Delving Deeper into the Formation of NaCl
The formation of sodium chloride is a classic example of an ionic reaction. Sodium, an alkali metal, readily loses one electron to achieve a stable electron configuration. Chlorine, a halogen, readily gains one electron to achieve a stable configuration. This electron transfer results in the formation of positively charged sodium ions (Na⁺) and negatively charged chloride ions (Cl⁻). The electrostatic attraction between these oppositely charged ions leads to the formation of the ionic compound, sodium chloride.
Distinguishing Salt from Mixtures
It's essential to distinguish salt from mixtures that might appear similar. For instance, seawater is a mixture containing salt dissolved in water. While seawater might seem like pure salt, it's a solution of various salts, minerals, and other substances. The proportion of salt in seawater can vary depending on location and other factors. In contrast, pure sodium chloride (table salt) is a chemically defined compound with a constant composition.
The Importance of Chemical Classification
The classification of substances like salt is fundamental to chemistry and other scientific disciplines. Accurate classification allows scientists to:
-
Predict Properties: Understanding whether a substance is an element, compound, or mixture allows scientists to predict its physical and chemical properties. This is crucial for various applications, from material science to drug development.
-
Control Reactions: Knowing the chemical composition of substances helps to control and optimize chemical reactions. This is essential in industrial processes, such as manufacturing and refining.
-
Understand Biological Processes: The classification of chemical compounds is essential for understanding biological processes. Many biological processes rely on specific chemical reactions involving elements and compounds.
-
Develop New Materials: Understanding the nature of elements and compounds allows scientists to design and develop new materials with specific properties. This is crucial in areas such as nanotechnology and materials engineering.
Beyond Table Salt: Other Types of Salts
While table salt (NaCl) is the most common type, numerous other salts exist. These salts share the characteristic of being ionic compounds formed from a cation (positive ion) and an anion (negative ion). Examples include:
- Potassium chloride (KCl): Used as a salt substitute and in fertilizers.
- Calcium chloride (CaCl₂): Used as a de-icer and in various industrial applications.
- Magnesium sulfate (MgSO₄): Known as Epsom salt, used in bath salts and as a laxative.
All these substances are compounds, not mixtures or elements, exhibiting the characteristic features of ionic bonding and fixed ratios of their constituent ions.
Conclusion: Salt – A Pure Compound
In conclusion, salt, or sodium chloride (NaCl), is unequivocally a compound. It's a pure substance formed from the chemical combination of sodium and chlorine atoms in a fixed 1:1 ratio, held together by strong ionic bonds. Its properties are distinctly different from those of its constituent elements. Understanding this fundamental classification highlights the importance of chemical principles in various fields, impacting our understanding of the world around us. The accurate classification of substances is vital for scientific progress and technological advancements. From culinary applications to industrial processes and biological functions, salt's role as a compound profoundly influences our lives.
Latest Posts
Latest Posts
-
3 By 3 System Of Equations Solver
Mar 18, 2025
-
What Number Is A Multiple Of 6
Mar 18, 2025
-
What Are The Equivalent Fractions Of 4 5
Mar 18, 2025
-
Which Pair Of Lines Are Parallel
Mar 18, 2025
-
Single Replacement Reaction Examples Real Life
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Salt A Compound Mixture Or Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
