What Is The Electron Configuration For Rb
Juapaving
Mar 28, 2025 · 6 min read
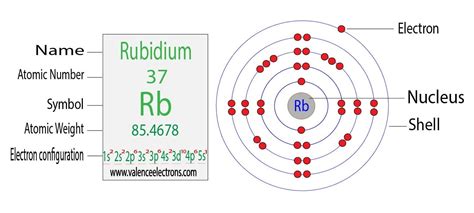
Table of Contents
What is the Electron Configuration for Rb? A Deep Dive into Rubidium's Atomic Structure
Rubidium (Rb), a fascinating alkali metal, holds a unique place in the periodic table. Understanding its electron configuration is key to comprehending its chemical properties, reactivity, and behavior. This comprehensive guide will delve into the electron configuration of rubidium, explaining the underlying principles, methods for determining it, and its implications.
Understanding Electron Configuration
Before we dive into rubidium's specific configuration, let's establish a foundational understanding of what electron configuration represents. An electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement dictates how an atom will interact with other atoms, forming chemical bonds and exhibiting characteristic properties. It follows the Aufbau principle, which states that electrons fill the lowest energy levels first.
The electron configuration is represented using a notation system that specifies the principal energy level (n), the subshell (s, p, d, or f), and the number of electrons in each subshell. For example, 1s² signifies two electrons in the 1s subshell.
Key Principles Governing Electron Configuration
Several crucial principles govern electron configuration:
- Aufbau Principle: Electrons occupy the lowest energy levels available first.
- Pauli Exclusion Principle: A maximum of two electrons can occupy a single orbital, and they must have opposite spins.
- Hund's Rule: Electrons will individually occupy each orbital within a subshell before pairing up. This maximizes the total spin.
Determining the Electron Configuration of Rubidium (Rb)
Rubidium has an atomic number of 37, meaning it possesses 37 protons and, in a neutral atom, 37 electrons. To determine its electron configuration, we systematically fill the electron shells according to the Aufbau principle.
The order of filling orbitals is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... and so on. However, a simpler and more intuitive way is to use the periodic table as a guide.
Using the Periodic Table to Determine Electron Configuration
The periodic table is a powerful tool for predicting electron configurations. Each period (row) corresponds to a principal energy level, and the blocks (s, p, d, f) indicate the subshells being filled.
-
Start with the first period: This period contains only the 1s subshell, which can hold a maximum of two electrons. Therefore, hydrogen (H) is 1s¹, and helium (He) is 1s².
-
Move to the second period: This period adds the 2s and 2p subshells. The 2s subshell holds two electrons (Li, Be), and the 2p subshell holds six (B, C, N, O, F, Ne).
-
Continue across the periods: Follow this pattern, adding electrons to the subshells according to their order in the periodic table and their electron capacities.
-
Reaching Rubidium: By following this systematic approach across the periodic table, we arrive at rubidium (Rb), element 37. Its electron configuration is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s¹.
Detailed Explanation of Rubidium's Electron Configuration: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s¹
Let's break down Rubidium's electron configuration step-by-step:
- 1s²: The first shell (n=1) contains only the s subshell, which holds two electrons.
- 2s²2p⁶: The second shell (n=2) has an s subshell (two electrons) and a p subshell (six electrons).
- 3s²3p⁶: The third shell (n=3) also contains an s subshell (two electrons) and a p subshell (six electrons).
- 4s²3d¹⁰: The fourth shell (n=4) begins with the 4s subshell (two electrons). Importantly, the 3d subshell (ten electrons) fills after the 4s subshell due to subtle energy level differences.
- 4p⁶: The fourth shell continues with the 4p subshell (six electrons).
- 5s¹: Finally, the fifth shell (n=5) begins with a single electron in the 5s subshell. This lone electron in the outermost shell is responsible for rubidium's characteristic reactivity.
Noble Gas Configuration and Rubidium
For conciseness, electron configurations are often written using noble gas notation. Noble gases are elements with completely filled outermost electron shells, making them exceptionally stable. To use noble gas notation, you replace the inner electron shells with the symbol of the preceding noble gas. For rubidium, the preceding noble gas is krypton (Kr), which has an electron configuration of 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶. Therefore, the noble gas configuration for rubidium is [Kr]5s¹.
Implications of Rubidium's Electron Configuration
Rubidium's electron configuration directly influences its chemical and physical properties. The single electron in the 5s subshell is readily lost, giving rubidium a +1 oxidation state. This easily lost electron is the reason why rubidium is highly reactive, readily forming ionic compounds with nonmetals and reacting vigorously with water. It's a characteristic shared by all alkali metals.
Reactivity and Chemical Bonding
The lone electron in the outermost shell makes rubidium a highly reactive element. It readily loses this electron to achieve a stable octet configuration, resembling the nearest noble gas, krypton. This electron loss leads to the formation of Rb⁺ ions, which readily form ionic bonds with electronegative elements like halogens (e.g., chlorine, bromine) and oxygen.
Physical Properties and Applications
The electron configuration also plays a role in rubidium's physical properties, such as its low ionization energy (the energy required to remove an electron), low melting and boiling points, and its characteristic metallic luster. These properties lead to various applications, although rubidium's high reactivity limits its widespread use. Some applications include specialized atomic clocks, photocells, and some niche applications in medicine and chemical research.
Beyond the Basics: Excited States and Electron Transitions
The electron configuration described above represents rubidium in its ground state – the lowest energy state. However, when energy is supplied (e.g., through heating or exposure to light), electrons can jump to higher energy levels, resulting in an excited state. These transitions are crucial for understanding rubidium's spectroscopic properties, including its characteristic emission and absorption spectra. These spectral lines are unique to rubidium and used in its identification.
Spectroscopy and Rubidium
The electron transitions between energy levels within rubidium atoms give rise to characteristic spectral lines. These spectral lines are the basis of spectroscopic techniques used to detect and quantify rubidium in various samples. The analysis of these spectra provides valuable information about the energy levels and electron configuration of the atom.
Conclusion
Understanding the electron configuration of rubidium is fundamental to comprehending its chemical and physical behavior. Its 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s¹ configuration, or more concisely [Kr]5s¹, explains its reactivity, its tendency to form +1 ions, and its place as a highly reactive alkali metal. The principles of the Aufbau principle, Pauli exclusion principle, and Hund's rule are crucial in determining this configuration and extrapolating to other elements. The implications of this configuration extend to various applications of rubidium in science and technology, underscoring the importance of understanding atomic structure. Further exploration into excited states and spectroscopy reveals even more intricate details of rubidium's atomic behavior.
Latest Posts
Latest Posts
-
What Type Of Heat Transfer Is Boiling Water
Mar 31, 2025
-
Lcm Of 5 3 And 6
Mar 31, 2025
-
In Which Hemisphere India Is Located
Mar 31, 2025
-
Worksheet On Passive And Active Voice
Mar 31, 2025
-
A Piece Of Land Surrounded By Water On Three Sides
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Rb . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
