What Is The Difference In Heat And Temperature
Juapaving
Mar 30, 2025 · 6 min read
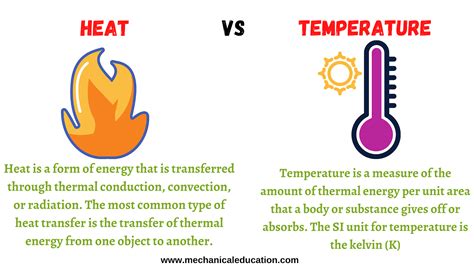
Table of Contents
What's the Difference Between Heat and Temperature?
Heat and temperature are often used interchangeably in everyday conversation, but in physics, they represent distinct concepts. Understanding the difference is crucial for comprehending various physical phenomena, from thermodynamics to climate change. This article will delve deep into the distinction between heat and temperature, exploring their definitions, measurement, and practical implications.
Defining Heat and Temperature
Temperature, at its core, measures the average kinetic energy of the particles (atoms and molecules) within a substance. Kinetic energy refers to the energy of motion. The faster the particles are moving, the higher the temperature. It's a measure of how hot or cold something is relative to a standard scale. Think of it as the intensity of thermal energy. We typically measure temperature using scales like Celsius, Fahrenheit, and Kelvin.
Heat, on the other hand, is the transfer of thermal energy between objects or systems at different temperatures. It's the energy itself, flowing from a hotter object to a colder object until thermal equilibrium—a state where both objects have the same temperature—is reached. Heat is not a property of a single object, but rather a process or a form of energy in transit. It's the quantity of thermal energy. We usually measure heat in Joules (J) or calories (cal).
Think of it like this: temperature is like the pressure in a water tank, while heat is like the water flowing from a higher-pressure tank to a lower-pressure tank. You can have high pressure (high temperature) in a small tank (small object), or low pressure (low temperature) in a large tank (large object). The actual flow of water (heat) depends on the pressure difference (temperature difference) and the size of the pipes (thermal conductivity).
The Mechanisms of Heat Transfer
Heat transfer occurs through three primary mechanisms:
1. Conduction:
Conduction is the transfer of heat through direct contact between particles. When one part of an object is heated, the particles in that area gain kinetic energy and vibrate more vigorously. These vibrations are passed on to neighboring particles, gradually transferring heat throughout the object. Metals are excellent conductors because their free electrons facilitate this energy transfer efficiently. Insulators, such as wood or plastic, impede heat conduction because their electrons are tightly bound to their atoms.
2. Convection:
Convection involves the transfer of heat through the movement of fluids (liquids or gases). When a fluid is heated, its density decreases, causing it to rise. Cooler, denser fluid then sinks to replace the warmer fluid, creating a cycle of movement that distributes heat. This is evident in boiling water, where hotter water rises to the surface while cooler water sinks to be heated. Atmospheric circulation and ocean currents are large-scale examples of convective heat transfer.
3. Radiation:
Radiation is the transfer of heat through electromagnetic waves. Unlike conduction and convection, radiation doesn't require a medium to travel. The sun's heat reaches Earth through radiation, as does the heat emitted by a glowing light bulb. All objects emit thermal radiation, with hotter objects emitting more radiation at shorter wavelengths. This is described by the Stefan-Boltzmann Law, which states that the power radiated per unit area is proportional to the fourth power of the absolute temperature.
Measuring Heat and Temperature
Temperature is measured using various instruments, including:
- Thermometers: These devices utilize the thermal expansion of liquids (like mercury or alcohol) or the change in electrical resistance of metals to measure temperature.
- Thermocouples: These consist of two dissimilar metals joined at their ends. A voltage is generated at the junction that is proportional to the temperature difference.
- Infrared thermometers: These measure the infrared radiation emitted by an object to determine its temperature without physical contact.
Heat, being a form of energy, is typically measured indirectly by observing its effects on a system. Calorimetry, for example, involves measuring the temperature change of a known mass of water to determine the amount of heat transferred. The specific heat capacity of the substance plays a crucial role in these calculations; it describes the amount of heat required to raise the temperature of one unit of mass by one degree.
The Relationship Between Heat and Temperature Change
The amount of heat (Q) required to change the temperature of a substance is directly proportional to its mass (m), specific heat capacity (c), and the temperature change (ΔT). This relationship is expressed by the equation:
Q = mcΔT
This equation highlights the interplay between heat and temperature. A larger mass requires more heat to achieve the same temperature change. Substances with higher specific heat capacities require more heat to raise their temperature by a given amount.
Practical Implications and Examples
Understanding the difference between heat and temperature has numerous practical implications in various fields:
- Meteorology: Weather forecasting relies heavily on temperature and heat transfer mechanisms to predict weather patterns. Understanding convection currents, for example, is essential for understanding cloud formation and storm development.
- Engineering: Engineers use this knowledge in designing efficient heating and cooling systems, optimizing engine performance, and developing new materials with specific thermal properties.
- Cooking: Cooking involves manipulating heat transfer to achieve desired outcomes. Conduction is used when cooking on a stovetop, while convection is utilized in ovens and microwaves.
- Medicine: Maintaining body temperature is crucial for human health. Fever, for instance, represents an increase in body temperature due to the body's response to infection, and understanding heat transfer is key to managing it.
- Climate Science: Climate change is driven by changes in the Earth's energy balance, involving heat transfer mechanisms on a global scale. Understanding greenhouse gases and their effects on radiative heat transfer is crucial for understanding the impacts of climate change.
Beyond the Basics: Specific Heat Capacity and Latent Heat
While the equation Q = mcΔT is fundamental, it only applies to changes in temperature without a change in phase (solid, liquid, gas). When a substance changes phase (e.g., ice melting into water), heat energy is absorbed or released, even though the temperature remains constant. This heat is called latent heat. Latent heat of fusion is involved in melting and freezing, while latent heat of vaporization is involved in boiling and condensation.
Specific heat capacity, as mentioned earlier, is a crucial property influencing the relationship between heat and temperature change. Different substances have different specific heat capacities. Water, for instance, has a remarkably high specific heat capacity, meaning it can absorb a significant amount of heat with a relatively small temperature increase. This is why water is often used as a coolant.
Conclusion: A Clear Distinction
While often used synonymously in everyday language, heat and temperature represent fundamentally distinct concepts in physics. Temperature measures the average kinetic energy of particles, reflecting the "hotness" or "coldness" of a substance, while heat describes the transfer of thermal energy between objects at different temperatures. Understanding their difference, the mechanisms of heat transfer, and their quantitative relationships is paramount for comprehending various natural phenomena and technological applications. From designing efficient engines to understanding climate change, a grasp of these concepts is essential for advancements in numerous fields.
Latest Posts
Latest Posts
-
Is 35 A Multiple Of 6
Apr 01, 2025
-
Exponents Worksheets Pdf With Answers 7th
Apr 01, 2025
-
Is Chlorine A Pure Substance Or Mixture
Apr 01, 2025
-
How Do You Write 19 In Roman Numerals
Apr 01, 2025
-
Why Is It Warmer When It Snows
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference In Heat And Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
