What Is The Difference Between Absorption And Emission Spectrum
Juapaving
Apr 01, 2025 · 6 min read
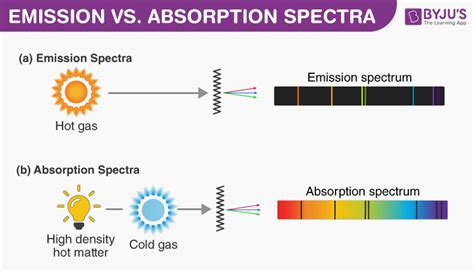
Table of Contents
What's the Difference Between Absorption and Emission Spectra?
Understanding the difference between absorption and emission spectra is fundamental to various fields, including astronomy, chemistry, and material science. Both are powerful tools used to identify the composition and properties of matter, but they reveal information through opposite processes. This article will delve deep into the intricacies of these spectra, clarifying their differences, explaining their underlying principles, and exploring their applications.
The Fundamentals: Light and Energy Levels
Before diving into the specifics of absorption and emission, it's crucial to understand the relationship between light and the energy levels within atoms and molecules. Atoms and molecules exist in discrete energy states. Electrons occupy specific orbitals, each associated with a particular energy level. The lowest energy level is called the ground state. When an atom or molecule absorbs energy, an electron can jump to a higher energy level, an excited state. Conversely, when an electron transitions from a higher energy level to a lower one, it releases energy, often in the form of light.
The energy of the light emitted or absorbed is directly related to the energy difference between the two levels involved in the transition. This relationship is described by the equation:
ΔE = hν
where:
- ΔE is the energy difference between the two levels
- h is Planck's constant (6.626 x 10^-34 Js)
- ν (nu) is the frequency of the light
Since the speed of light (c) and wavelength (λ) are related by c = λν, we can also write the equation as:
ΔE = hc/λ
This equation highlights that each energy transition corresponds to a specific wavelength (and frequency) of light. This is the key to understanding both absorption and emission spectra.
Absorption Spectra: What Atoms and Molecules Absorb
An absorption spectrum is created when light passes through a sample of atoms or molecules. Specific wavelengths of light are absorbed by the sample as electrons transition to higher energy levels. The resulting spectrum shows dark lines or bands at the wavelengths corresponding to the absorbed light, against a bright background of the original light source. The pattern of these dark lines or bands is unique to the specific substance being analyzed.
How Absorption Spectra are Formed: A Step-by-Step Explanation
- Light Source: A continuous source of light, such as a tungsten lamp or a synchrotron, emits light across a broad range of wavelengths.
- Sample Interaction: This light then passes through a sample containing atoms or molecules.
- Absorption: If the energy of a photon in the incident light matches the energy difference between two energy levels in the atoms or molecules, the photon is absorbed. This causes an electron to transition to a higher energy level.
- Transmission: The light that is not absorbed passes through the sample.
- Detection: A detector, such as a spectrometer, measures the intensity of the transmitted light at various wavelengths.
- Spectrum Generation: The resulting spectrum shows decreased intensity (dark lines or bands) at the wavelengths that were absorbed.
Key Characteristics of Absorption Spectra:
- Dark lines or bands: Indicate the wavelengths of light absorbed.
- Unique fingerprint: The pattern of absorption lines is unique to each substance, acting like a "fingerprint" for identification.
- Dependent on the sample: The absorption spectrum changes depending on the concentration and physical state of the sample.
Emission Spectra: What Atoms and Molecules Emit
An emission spectrum is produced when excited atoms or molecules release energy in the form of light. This happens when electrons transition from higher energy levels back to lower ones. The resulting spectrum shows bright lines or bands at the wavelengths corresponding to the emitted light, against a dark background. Like absorption spectra, the pattern of these bright lines is unique to each substance.
How Emission Spectra are Formed: A Step-by-Step Explanation
- Excitation: Atoms or molecules are excited by providing them with energy. This can be done through various methods, such as heating, electrical discharge, or irradiation with high-energy light.
- Excited State: Electrons jump to higher energy levels.
- Relaxation: Electrons are inherently unstable in excited states and quickly return to lower energy levels.
- Photon Emission: As electrons transition back to lower energy levels, they release energy in the form of photons (light).
- Detection: The emitted light is then detected by a spectrometer.
- Spectrum Generation: The resulting spectrum shows bright lines at the wavelengths corresponding to the emitted photons.
Key Characteristics of Emission Spectra:
- Bright lines or bands: Indicate the wavelengths of light emitted.
- Unique fingerprint: The pattern of emission lines is unique to each substance.
- Dependent on excitation method: The intensity of emission lines can vary depending on the method used to excite the atoms or molecules.
The Relationship Between Absorption and Emission Spectra
Absorption and emission spectra are closely related, reflecting the same energy level transitions within atoms and molecules. For a given element or molecule, the wavelengths of light absorbed in the absorption spectrum correspond precisely to the wavelengths of light emitted in the emission spectrum. This is because the energy difference between the same two energy levels is identical, whether the transition involves absorption or emission.
This relationship is often depicted using energy level diagrams, which visually represent the energy levels of an atom or molecule and the transitions between them. The difference lies in the direction of the transition: upward for absorption and downward for emission.
Applications of Absorption and Emission Spectroscopy
Both absorption and emission spectroscopy are widely used in various fields due to their ability to provide detailed information about the composition and properties of matter. Here are some key applications:
Absorption Spectroscopy Applications:
- Astronomy: Analyzing the absorption spectra of stars and galaxies allows astronomers to determine their chemical composition and temperature.
- Chemistry: Determining the concentration of substances in solutions through techniques like UV-Vis spectroscopy.
- Medicine: Diagnosing certain medical conditions through the absorption of light by blood or tissue samples.
- Environmental Science: Monitoring pollutants in air and water using absorption spectroscopy techniques.
Emission Spectroscopy Applications:
- Astronomy: Identifying the elements present in stars, nebulae, and other celestial bodies based on their emission spectra.
- Forensic Science: Analyzing the emission spectra of trace evidence to identify substances and materials.
- Material Science: Characterizing the properties of materials based on their emission characteristics.
- Geochemistry: Analyzing the composition of rocks and minerals using emission spectroscopy techniques.
Differences Summarized: A Table for Clarity
| Feature | Absorption Spectrum | Emission Spectrum |
|---|---|---|
| Process | Light passes through a sample; some wavelengths are absorbed. | Excited atoms/molecules emit light. |
| Appearance | Dark lines/bands on a bright background | Bright lines/bands on a dark background |
| Energy Change | Electrons absorb energy and jump to higher energy levels. | Electrons release energy and jump to lower energy levels. |
| Information Provided | Identifies what wavelengths are absorbed, indicating the presence of specific substances. | Identifies what wavelengths are emitted, providing information about the excited states and transitions in atoms or molecules. |
Conclusion: Powerful Tools for Analysis
Absorption and emission spectra are invaluable analytical tools. While they represent opposite processes—absorption and emission of light—they provide complementary information about the same fundamental atomic and molecular transitions. Understanding the differences and relationships between these spectra is essential for effectively applying them in diverse scientific fields, from unraveling the mysteries of the cosmos to developing new materials and technologies. The unique "fingerprint" provided by each type of spectrum makes them indispensable for identification and analysis across many disciplines.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 2 And 8
Apr 02, 2025
-
Greatest Amount Of Digestion Takes Place In The
Apr 02, 2025
-
What Is Bigger 1 2 Or 3 8
Apr 02, 2025
-
Least Common Multiple Of 20 And 30
Apr 02, 2025
-
Difference Between Starch Cellulose And Glycogen
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Absorption And Emission Spectrum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
