What Is The Difference Between A Nucleotide And A Nucleoside
Juapaving
Mar 23, 2025 · 5 min read
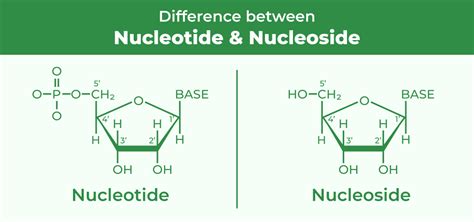
Table of Contents
What's the Difference Between a Nucleotide and a Nucleoside? A Deep Dive into Nucleic Acid Building Blocks
Understanding the fundamental building blocks of life is crucial for grasping the complexities of biology. Nucleic acids, the carriers of genetic information, are composed of smaller units: nucleotides and nucleosides. While these terms are often used interchangeably, there's a crucial distinction that determines their roles in the intricate machinery of cells. This article will delve into the precise differences between nucleotides and nucleosides, exploring their structures, functions, and significance in various biological processes.
Defining Nucleosides: The Sugar-Base Duo
A nucleoside is a relatively simple molecule composed of two parts: a nitrogenous base and a five-carbon sugar (pentose). Think of it as the foundational element before further modification. Let's break down each component:
1. Nitrogenous Bases: The Information Carriers
Nitrogenous bases are cyclic organic molecules containing nitrogen atoms. They are categorized into two main groups:
-
Purines: These are larger, double-ringed structures. Adenine (A) and guanine (G) are the purine bases found in both DNA and RNA.
-
Pyrimidines: These are smaller, single-ringed structures. Cytosine (C) is found in both DNA and RNA, while thymine (T) is specific to DNA, and uracil (U) is specific to RNA.
The specific arrangement of these bases along the nucleic acid chain dictates the genetic code. The hydrogen bonding between complementary base pairs (A with T or U, and G with C) is essential for the double helix structure of DNA and the secondary structures of RNA.
2. Pentose Sugars: The Structural Backbone
The pentose sugar acts as the backbone to which the nitrogenous base is attached. There are two types of pentose sugars:
-
Ribose: Found in RNA (ribonucleic acid). It has a hydroxyl (-OH) group attached to the 2' carbon atom.
-
Deoxyribose: Found in DNA (deoxyribonucleic acid). It lacks the hydroxyl (-OH) group at the 2' carbon atom; hence the "deoxy" prefix. This subtle difference significantly impacts the stability and structure of DNA compared to RNA.
The glycosidic bond connects the nitrogenous base to the 1' carbon of the pentose sugar, forming the nucleoside. For example, adenine + ribose = adenosine, and adenine + deoxyribose = deoxyadenosine. The same principle applies to the other bases.
Defining Nucleotides: Phosphate Powerhouse
Nucleotides are the true workhorses of nucleic acid structure and function. They represent the next level of complexity, building upon the nucleoside foundation. A nucleotide is composed of three parts:
-
Nitrogenous Base: The same purine or pyrimidine base found in nucleosides.
-
Pentose Sugar: Ribose in RNA nucleotides and deoxyribose in DNA nucleotides.
-
Phosphate Group: This is the key differentiator between a nucleoside and a nucleotide. A phosphate group (PO₄³⁻) is attached to the 5' carbon atom of the pentose sugar via a phosphoester bond. This phosphate group is crucial for the energy transfer and linkage functions of nucleotides.
The phosphate group's presence significantly alters the molecule's properties. It introduces a negative charge, making nucleotides highly polar and soluble in water. This solubility is essential for their movement and interaction within the aqueous cellular environment. Furthermore, the phosphate group plays a vital role in the formation of the phosphodiester bonds that link nucleotides together to form the polynucleotide chains of DNA and RNA.
The Role of Phosphates in Nucleic Acid Structure
The phosphate group isn't merely an addition; it's the linchpin of nucleic acid construction. The 5'-phosphate group of one nucleotide forms a phosphodiester bond with the 3'-hydroxyl group of the next nucleotide. This linkage creates the sugar-phosphate backbone, the structural framework of DNA and RNA. The directionality of this backbone (5' to 3') is crucial for DNA replication and transcription.
The multiple phosphate groups in nucleotides also contribute to their energy storage capacity. Adenosine triphosphate (ATP), the primary energy currency of cells, is a nucleotide with three phosphate groups. The high-energy bonds between these phosphates provide the energy for countless cellular processes. Similarly, guanosine triphosphate (GTP), cytidine triphosphate (CTP), and uridine triphosphate (UTP) have similar roles in various metabolic pathways.
Nucleotides Beyond Nucleic Acids: Versatile Molecules
While nucleotides are best known for their role in DNA and RNA, their functions extend far beyond the realm of genetic information. They serve as crucial components in various cellular processes:
-
Signal Transduction: Cyclic AMP (cAMP) and cyclic GMP (cGMP), cyclic nucleotides, act as second messengers in signal transduction pathways, relaying information from cell surface receptors to intracellular targets.
-
Enzyme Cofactors: Certain nucleotides, like nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD), act as coenzymes in metabolic reactions, facilitating electron transfer.
-
Metabolic Intermediates: Nucleotides are involved in various metabolic pathways as precursors or intermediates in the synthesis of other essential molecules.
Key Differences Summarized: Nucleosides vs. Nucleotides
To reiterate the fundamental differences, let's summarize them in a table:
| Feature | Nucleoside | Nucleotide |
|---|---|---|
| Components | Nitrogenous base + Sugar | Nitrogenous base + Sugar + Phosphate |
| Phosphate Group | Absent | Present |
| Charge | Neutral | Negatively charged |
| Solubility | Relatively less soluble | Highly soluble |
| Function | Precursor to nucleotides | Building blocks of nucleic acids, energy carriers, enzyme cofactors |
Conclusion: Understanding the Building Blocks
The distinction between nucleosides and nucleotides is crucial for comprehending the complexity of cellular processes. While nucleosides provide the basic framework, it is the addition of the phosphate group in nucleotides that unlocks their vast functional potential. Understanding these differences lays the groundwork for appreciating the intricate roles of nucleic acids in genetics, metabolism, and cellular signaling. The seemingly simple addition of a phosphate group transforms a nucleoside into a nucleotide, a powerhouse of biological activity. This seemingly small difference underscores the beauty and precision of molecular biology. Further research into these building blocks promises to continue unveiling the deeper secrets of life.
Latest Posts
Latest Posts
-
Which Of The Following Is An Example Of Radiation
Mar 24, 2025
-
A Line That Intersects A Circle In Exactly One Point
Mar 24, 2025
-
Is Boiling An Endothermic Or Exothermic Process
Mar 24, 2025
-
What Is Resistance To Motion Called
Mar 24, 2025
-
Kirchhoffs Junction Rule Is A Statement Of
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between A Nucleotide And A Nucleoside . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
