What Is The Chemical Formula For Protein
Juapaving
Mar 29, 2025 · 7 min read
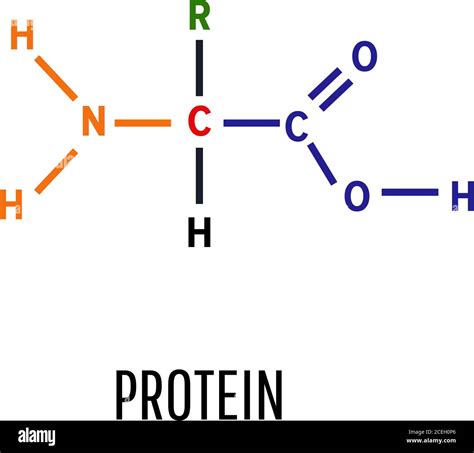
Table of Contents
What is the Chemical Formula for Protein? A Deep Dive into the Building Blocks of Life
Proteins are the workhorses of our cells, responsible for a vast array of functions crucial for life. From catalyzing biochemical reactions to providing structural support, their roles are diverse and indispensable. But what exactly is a protein, chemically speaking? There isn't a single, simple chemical formula that encapsulates all proteins, unlike, say, water (H₂O) or table salt (NaCl). This is because proteins are incredibly diverse macromolecules, with an astounding array of structures and functions. Understanding their chemical makeup requires exploring their fundamental building blocks: amino acids.
The Amino Acid Alphabet: The Foundation of Protein Structure
Proteins are polymers, meaning they're large molecules composed of repeating smaller units. These smaller units, in the case of proteins, are amino acids. There are 20 standard amino acids, each with a unique chemical structure, that serve as the "letters" of the protein "alphabet". The sequence of these amino acids, dictated by our genes, determines a protein's three-dimensional structure and, ultimately, its function.
Each amino acid shares a common core structure:
- A central carbon atom (α-carbon): This carbon atom is bonded to four different groups.
- An amino group (-NH₂): This is a basic group, meaning it can accept a proton (H⁺).
- A carboxyl group (-COOH): This is an acidic group, meaning it can donate a proton (H⁺).
- A hydrogen atom (-H): A simple hydrogen atom.
- A side chain (R-group): This is the variable group that distinguishes one amino acid from another. The R-group can be anything from a simple hydrogen atom (as in glycine) to a complex aromatic ring (as in tryptophan).
The Diversity of R-Groups: Defining Amino Acid Properties
The R-group is the key to understanding the diversity of amino acids. These side chains impart unique chemical properties to each amino acid, influencing how they interact with each other and their environment. These properties can be broadly categorized as:
-
Nonpolar (hydrophobic): These R-groups are repelled by water and tend to cluster together in the interior of proteins. Examples include alanine, valine, leucine, isoleucine, methionine, phenylalanine, tryptophan, and proline. Proline is unique because its R-group forms a ring structure, connecting back to the amino group.
-
Polar (hydrophilic): These R-groups are attracted to water and are often found on the surface of proteins, interacting with the aqueous environment. Examples include serine, threonine, cysteine, tyrosine, asparagine, and glutamine. Cysteine has a sulfhydryl group (-SH) that can form disulfide bonds, crucial for stabilizing protein structure.
-
Charged (hydrophilic): These R-groups carry a net positive or negative charge at physiological pH. Positively charged amino acids (basic) include lysine, arginine, and histidine. Negatively charged amino acids (acidic) include aspartic acid and glutamic acid.
This diversity in R-groups is crucial for the vast array of protein structures and functions. The unique properties of each amino acid dictate how they interact with each other, influencing the protein's folding and ultimate 3D structure.
Peptide Bonds: Linking Amino Acids into Chains
Amino acids are joined together by peptide bonds to form polypeptide chains. A peptide bond is a covalent bond formed between the carboxyl group of one amino acid and the amino group of the next amino acid. This reaction involves the removal of a water molecule (dehydration synthesis). A polypeptide chain is simply a sequence of amino acids linked by peptide bonds. Proteins can be composed of one or more polypeptide chains.
Protein Structure: From Primary to Quaternary
The structure of a protein is intimately linked to its function. Protein structure is often described in four levels:
1. Primary Structure: The Amino Acid Sequence
The primary structure of a protein refers to the linear sequence of amino acids in its polypeptide chain(s). This sequence is dictated by the genetic code and is crucial for determining the higher-order structures and ultimately the protein's function. Even a single amino acid change can drastically alter a protein's properties.
2. Secondary Structure: Local Folding Patterns
The secondary structure refers to local folding patterns within the polypeptide chain. These patterns are stabilized by hydrogen bonds between the backbone atoms of the amino acids. Common secondary structures include:
- α-helices: A right-handed coiled structure stabilized by hydrogen bonds between every fourth amino acid.
- β-sheets: Extended regions of polypeptide chains arranged side-by-side, stabilized by hydrogen bonds between adjacent chains. These can be parallel (chains run in the same direction) or antiparallel (chains run in opposite directions).
- Loops and turns: Regions connecting α-helices and β-sheets.
3. Tertiary Structure: The 3D Arrangement
The tertiary structure describes the overall three-dimensional arrangement of a polypeptide chain. This structure is determined by various interactions between the amino acid side chains, including:
- Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from water.
- Hydrogen bonds: Between polar side chains.
- Ionic bonds (salt bridges): Between oppositely charged side chains.
- Disulfide bonds: Covalent bonds between cysteine residues.
The tertiary structure is crucial for the protein's function, as it brings specific amino acid side chains into close proximity, creating active sites for enzymes or binding sites for other molecules.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins are composed of multiple polypeptide chains, each with its own tertiary structure. The arrangement of these individual chains is known as the quaternary structure. These subunits often interact through the same forces that stabilize tertiary structure: hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bonds. Hemoglobin, for example, has a quaternary structure composed of four polypeptide chains.
The Chemical Formula Conundrum: Why There Isn't One
Given the complexity of protein structure and the vast diversity of amino acids and their arrangements, it's impossible to represent all proteins with a single chemical formula. The formula would need to vary dramatically depending on the specific amino acid sequence, length, and post-translational modifications of each protein.
Instead of a single formula, we describe proteins by their amino acid sequence, which is often represented using a three-letter or single-letter code for each amino acid. For example, a small peptide might be represented as Gly-Ala-Ser (Glycine-Alanine-Serine) using the three-letter code.
The molecular weight of a protein can be calculated based on its amino acid sequence, providing a more quantitative measure. However, even this is an approximation, as post-translational modifications (such as glycosylation or phosphorylation) can add to the protein's overall mass.
Beyond the Basic Structure: Post-Translational Modifications
The story doesn't end with the primary, secondary, tertiary, and quaternary structures. Proteins can undergo various post-translational modifications after they are synthesized. These modifications can alter the protein's function, stability, or localization within the cell. Some common post-translational modifications include:
- Glycosylation: The addition of carbohydrate groups.
- Phosphorylation: The addition of phosphate groups.
- Acetylation: The addition of acetyl groups.
- Ubiquitination: The addition of ubiquitin molecules, often targeting proteins for degradation.
These modifications significantly impact the overall chemical composition and properties of a protein.
Conclusion: A World of Protein Diversity
Understanding the chemical nature of proteins requires moving beyond the limitations of a single chemical formula. Proteins are incredibly diverse macromolecules with complex structures and functions. Their building blocks, the 20 standard amino acids, are linked together by peptide bonds to form polypeptide chains. These chains fold into intricate three-dimensional structures, influenced by a variety of interactions between amino acid side chains. Post-translational modifications add another layer of complexity, further diversifying the properties of these essential biological molecules. By appreciating the intricate interplay of amino acid sequence, structure, and modification, we can better understand the remarkable diversity and vital roles of proteins in all living organisms. This detailed understanding is crucial for numerous fields, including medicine, biotechnology, and materials science. Further research continues to unveil even more about the subtle nuances of protein structure and function, opening doors to exciting possibilities in various scientific domains.
Latest Posts
Latest Posts
-
Find Area Between Two Curves Calculator
Mar 31, 2025
-
Which Odor Best Describes The Smell Of Acetic Acid
Mar 31, 2025
-
Given A 120 Degree Angle Construct A 0 Degree Angl
Mar 31, 2025
-
Two Prime Numbers Which Differ By 2 Are Called
Mar 31, 2025
-
Two Angles Whose Measures Have A Sum Of 180
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Chemical Formula For Protein . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
