Write The Chemical Formula For Chloric Acid
Juapaving
Mar 17, 2025 · 6 min read
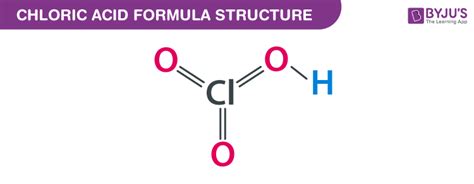
Table of Contents
Delving Deep into Chloric Acid: Formula, Properties, and Applications
Chloric acid, a potent oxidizing agent, holds a significant place in chemistry. Understanding its chemical formula is just the starting point for appreciating its diverse properties and applications. This comprehensive article delves into the intricacies of chloric acid, exploring its formula, production methods, characteristics, safety precautions, and wide-ranging uses.
The Chemical Formula: HClO₃
The chemical formula for chloric acid is HClO₃. This simple yet powerful formula encapsulates the compound's composition: one hydrogen atom (H), one chlorine atom (Cl), and three oxygen atoms (O). The structure reveals a central chlorine atom bonded to three oxygen atoms, with one oxygen atom also bonded to the hydrogen atom, forming the characteristic acidic hydrogen. This arrangement dictates the acid's behavior and reactivity. Understanding this fundamental formula is crucial for predicting its interactions with other substances.
Production Methods: Synthesizing Chloric Acid
While chloric acid isn't readily available commercially due to its instability, it can be synthesized through several chemical processes. These methods often involve indirect approaches, focusing on the production of chlorates which are then used to generate the acid.
1. From Barium Chlorate: A Classic Approach
Historically, a common method involved reacting barium chlorate (Ba(ClO₃)₂) with sulfuric acid (H₂SO₄). This reaction precipitates barium sulfate (BaSO₄), a relatively insoluble compound, leaving chloric acid in solution. The reaction can be represented as:
Ba(ClO₃)₂ + H₂SO₄ → 2HClO₃ + BaSO₄↓
The barium sulfate precipitate is then filtered out, leaving behind an aqueous solution of chloric acid. However, this method requires careful handling due to the corrosive nature of the reactants and the potential for contamination.
2. Electrolysis of Sodium Chloride: An Electrolytic Route
Electrolysis of a concentrated solution of sodium chloride (NaCl) can yield sodium chlorate (NaClO₃). Subsequent reaction with a strong acid, such as sulfuric acid, can then liberate chloric acid. This method is more complex and requires specialized electrochemical equipment. The process involves several steps and careful control of parameters to maximize the yield of chloric acid.
3. From Chlorine Dioxide: A Gas-Phase Reaction
Chlorine dioxide (ClO₂) can be reacted with water (H₂O) under specific conditions to produce a mixture of chloric acid and chlorous acid (HClO₂). This method requires careful control of reaction parameters to optimize the formation of chloric acid. Separating the two acids can be challenging.
Important Considerations in Production:
Regardless of the chosen method, the production of chloric acid necessitates meticulous safety precautions. The compound is inherently unstable and can decompose explosively under certain conditions. The process requires specialized equipment, skilled personnel, and a controlled environment to mitigate the risks associated with handling strong oxidizing agents.
Properties of Chloric Acid: A Deeper Look
Chloric acid boasts a unique set of properties that contribute to its reactivity and applications. These properties are intricately linked to its molecular structure and the strong oxidizing power of the chlorate ion (ClO₃⁻).
1. Physical Properties: Appearance and Behavior
In its pure form, chloric acid exists as a colorless, viscous liquid. However, it's highly unstable and prone to decomposition, making it difficult to isolate in pure form. It readily dissolves in water, forming an aqueous solution. The solution exhibits strong acidic properties, indicating its ability to donate protons (H⁺) in solution.
2. Chemical Properties: Reactivity and Oxidation
The most defining characteristic of chloric acid is its potent oxidizing ability. The chlorine atom in the chlorate ion carries a relatively high oxidation state (+5), making it a strong oxidizing agent. This property allows it to readily accept electrons from other substances, leading to oxidation-reduction (redox) reactions. These reactions often produce chlorine gas (Cl₂), oxygen gas (O₂), or other chlorine oxyanions depending on the reducing agent.
3. Instability and Decomposition: A Key Consideration
Chloric acid is notoriously unstable, particularly in concentrated form. It readily decomposes, even at moderate temperatures, releasing chlorine dioxide (ClO₂), a toxic and explosive gas. The decomposition reaction can be accelerated by heat, light, or the presence of catalysts. This inherent instability presents significant challenges in handling and storage.
Decomposition Reaction:
4HClO₃ → 4ClO₂ + 2H₂O + O₂ + 2Cl₂
This explosive potential necessitates careful handling and stringent safety measures when working with chloric acid.
Applications of Chloric Acid: Diverse Uses
Despite its instability, chloric acid finds applications in several chemical processes, primarily leveraging its strong oxidizing power.
1. Production of Chlorates: A Crucial Intermediate
Chloric acid serves as a crucial intermediate in the production of various chlorates, including potassium chlorate (KClO₃) and sodium chlorate (NaClO₃). These chlorates have various applications, including in the manufacturing of matches, fireworks, and bleaching agents. The acid's role in these processes highlights its significance in industrial chemistry.
2. Oxidizing Agent in Chemical Synthesis: A Powerful Tool
Its powerful oxidizing ability makes chloric acid useful in various chemical synthesis reactions. It can oxidize organic compounds and other substances, creating new functional groups or facilitating specific transformations. However, the inherent instability and potential for explosive decomposition necessitate careful control of reaction conditions.
3. Laboratory Reagent: A Specialized Use
In laboratory settings, chloric acid (often in dilute form) finds limited use as a reagent in certain analytical and research procedures. Its strong oxidizing power and acidity can be utilized in specific applications, but its instability remains a significant constraint.
Safety Precautions: Handling Chloric Acid Responsibly
Due to its inherent instability and oxidizing power, handling chloric acid demands stringent safety measures.
1. Protective Equipment: Essential Gear
Working with chloric acid necessitates the use of appropriate personal protective equipment (PPE), including safety goggles, gloves, lab coats, and respiratory protection. This safeguards against potential skin contact, eye irritation, and inhalation of hazardous vapors.
2. Controlled Environment: Minimizing Risks
The handling and storage of chloric acid should occur in a well-ventilated environment to minimize the risk of exposure to chlorine dioxide or other potentially hazardous decomposition products. The area should be free of flammable materials, as the acid's oxidizing properties increase the fire hazard.
3. Proper Storage: Safekeeping the Acid
Chloric acid should be stored in tightly sealed containers in a cool, dry place away from heat sources, direct sunlight, and incompatible materials. The containers should be made of materials resistant to corrosion by the acid.
4. Waste Disposal: Environmental Responsibility
Disposal of chloric acid and its waste products must follow strict environmental regulations. Neutralization procedures may be necessary to reduce the acidity and oxidizing potential before disposal. Always consult local environmental authorities for proper waste handling guidelines.
Conclusion: The Importance of Chloric Acid
Chloric acid, despite its instability, holds a significant position in chemistry. Understanding its chemical formula, production methods, properties, and applications allows us to appreciate its role in various chemical processes. However, its hazardous nature necessitates careful handling and stringent safety procedures. The future of chloric acid's use will likely involve focusing on safe and controlled applications where its oxidizing power can be effectively harnessed without compromising safety. The information detailed here underlines the importance of responsible scientific practice when dealing with this potent chemical compound.
Latest Posts
Latest Posts
-
Which Of The Following Is An Example Of A Decomposer
Mar 17, 2025
-
Highest Common Factor Of 24 And 36
Mar 17, 2025
-
What Goes Up And Down But Doesnt Move
Mar 17, 2025
-
Where Does Most Metabolic Activity In The Cell Occur
Mar 17, 2025
-
The Most Reactive Group Of The Nonmetals Is The
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Write The Chemical Formula For Chloric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
