What Is The Average Atomic Mass Of Boron
Juapaving
Mar 31, 2025 · 5 min read
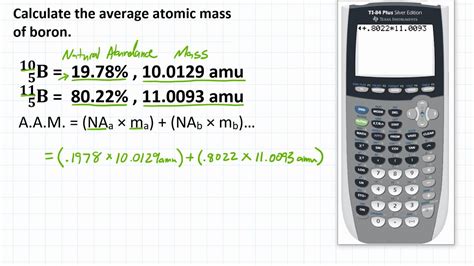
Table of Contents
What is the Average Atomic Mass of Boron? A Deep Dive into Isotopes and Atomic Weight
Boron, a metalloid element crucial in various applications from detergents to nuclear reactors, presents a fascinating case study in atomic mass. Understanding its average atomic mass requires delving into the concept of isotopes and their relative abundances. This article will provide a comprehensive explanation of boron's average atomic mass, exploring the underlying principles and their implications.
Understanding Atomic Mass
Before we focus on boron, let's establish a firm understanding of atomic mass. The atomic mass of an element represents the average mass of all its isotopes, weighted by their natural abundance. It's crucial to differentiate between atomic mass and mass number.
- Mass number: Represents the total number of protons and neutrons in an atom's nucleus. It's a whole number.
- Atomic mass: A weighted average reflecting the different isotopes and their prevalence in nature. It's usually a decimal number.
The difference arises because most elements exist as a mixture of isotopes – atoms of the same element with the same number of protons but differing numbers of neutrons. These isotopes have different mass numbers, contributing to the weighted average that we call the atomic mass.
Boron's Isotopes: The Key Players
Boron has two naturally occurring isotopes:
- Boron-10 (¹⁰B): This isotope possesses 5 protons and 5 neutrons.
- Boron-11 (¹¹B): This isotope has 5 protons and 6 neutrons.
The difference in neutron number results in a slight mass difference between these isotopes. This difference directly impacts the calculation of boron's average atomic mass. Understanding the relative abundance of each isotope is paramount for accurate calculation.
Calculating the Average Atomic Mass of Boron
The average atomic mass is calculated using the following formula:
Average Atomic Mass = (Mass of Isotope 1 × Abundance of Isotope 1) + (Mass of Isotope 2 × Abundance of Isotope 2) + ...
For boron, this translates to:
Average Atomic Mass (Boron) = (Mass of ¹⁰B × Abundance of ¹⁰B) + (Mass of ¹¹B × Abundance of ¹¹B)
The key data needed for this calculation is the precise mass of each isotope and their relative abundance in nature. While the mass number provides a reasonable approximation, the actual mass of each isotope is slightly different due to the binding energy within the nucleus. These precise masses are typically determined using highly sensitive mass spectrometry techniques.
The accepted values are approximately:
- Mass of ¹⁰B: 10.0129 amu (atomic mass units)
- Mass of ¹¹B: 11.0093 amu
- Abundance of ¹⁰B: Approximately 19.9% or 0.199
- Abundance of ¹¹B: Approximately 80.1% or 0.801
Now, we can plug these values into the formula:
Average Atomic Mass (Boron) = (10.0129 amu × 0.199) + (11.0093 amu × 0.801)
Average Atomic Mass (Boron) ≈ 1.99256 + 8.81733
Average Atomic Mass (Boron) ≈ 10.81 amu
This value (approximately 10.81 amu) is the average atomic mass of boron you'll find on the periodic table. It reflects the weighted average of the two naturally occurring isotopes based on their relative abundances. Small variations may exist depending on the source and precision of the measured isotopic abundances.
The Significance of Average Atomic Mass
The average atomic mass is crucial in numerous scientific and engineering applications:
- Stoichiometric calculations: Accurate determination of the average atomic mass is essential for precise stoichiometric calculations in chemistry, ensuring the correct proportions of reactants and products in chemical reactions.
- Nuclear reactions: Understanding isotopic abundances and masses is critical in nuclear physics and engineering, particularly in applications involving boron's role in nuclear reactors (neutron absorption).
- Materials science: Boron's unique properties make it a valuable component in various materials. Knowing its average atomic mass is crucial for predicting and controlling material properties.
- Analytical chemistry: Techniques like mass spectrometry rely on precise knowledge of isotopic masses and abundances for accurate elemental analysis.
Factors Affecting Isotopic Abundance and Average Atomic Mass
While the average atomic mass of boron remains relatively constant, subtle variations can occur depending on the source material. These variations stem from:
- Geographical location: The isotopic ratios of elements can differ slightly depending on their geological origins and the processes that have shaped the earth's crust. This is especially relevant for elements with multiple isotopes.
- Sample preparation: The methods used to prepare samples for analysis can subtly influence the measured isotopic ratios. Careful sample preparation techniques are essential to minimize such effects.
- Industrial processes: Certain industrial processes might inadvertently alter the isotopic ratios of elements. This is less common for naturally occurring boron but relevant in specialized applications.
Beyond Boron: A Broader Perspective
The principles discussed above regarding boron's average atomic mass apply equally to other elements with multiple isotopes. Many elements exhibit isotopic variations, each with its own unique mass and abundance. These variations contribute to the rich diversity of atomic weights found on the periodic table. Understanding these variations is essential for a complete grasp of atomic structure and its implications across various scientific disciplines.
Conclusion: A Weighted Average with Deep Implications
The average atomic mass of boron, approximately 10.81 amu, isn't just a number on a periodic table; it's a fundamental property reflecting the weighted average of its naturally occurring isotopes. This weighted average is essential for various calculations and applications ranging from basic stoichiometry to sophisticated nuclear engineering and materials science. The precision of this value relies heavily on the accurate determination of isotopic abundances and masses, showcasing the importance of advanced analytical techniques in modern science. Understanding the intricacies of isotopic abundances and the calculation of average atomic mass underscores the sophisticated nature of atomic structure and its far-reaching implications across numerous scientific and engineering fields. Furthermore, exploring the factors that can influence isotopic abundance provides valuable insights into geological processes and the subtle variations found in the elemental composition of our planet. This knowledge forms the basis for a deeper understanding of the natural world and enables more accurate predictions and control in various scientific and engineering endeavors.
Latest Posts
Latest Posts
-
What Type Of Device Is A Keyboard
Apr 02, 2025
-
Lcm Of 7 4 And 2
Apr 02, 2025
-
Why Is Dry Ice Called Dry Ice
Apr 02, 2025
-
An Apple Is Cut Physical Or Chemical
Apr 02, 2025
-
45 Minutes Is What Percent Of An Hour
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Average Atomic Mass Of Boron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
