What Is The Angle Of A Bent Molecule
Juapaving
Mar 24, 2025 · 5 min read
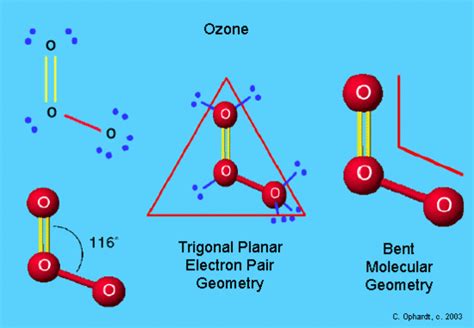
Table of Contents
What is the Angle of a Bent Molecule? A Deep Dive into Molecular Geometry
Understanding molecular geometry is crucial in chemistry, as it dictates a molecule's physical and chemical properties. One common molecular shape is the bent geometry, characterized by a central atom bonded to two other atoms with a bond angle less than 180 degrees. This article will delve into the intricacies of bent molecules, exploring the factors influencing their bond angles and providing examples to solidify understanding.
What Makes a Molecule Bent?
A bent molecule arises from the presence of lone pairs of electrons on the central atom. These lone pairs repel bonding pairs, compressing the bond angle between the attached atoms. The repulsion between electron pairs follows this order: lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair. This means that lone pairs exert a stronger repulsive force than bonding pairs, causing a greater deviation from the ideal bond angle.
VSEPR Theory: The Guiding Principle
The Valence Shell Electron Pair Repulsion (VSEPR) theory is the cornerstone of predicting molecular geometry. VSEPR theory postulates that electron pairs around a central atom arrange themselves to minimize repulsion, leading to specific geometric arrangements. For bent molecules, we typically encounter two bonding pairs and one or two lone pairs on the central atom.
Predicting Bond Angles in Bent Molecules
The ideal bond angles for various electron pair arrangements are as follows:
- Two bonding pairs, zero lone pairs (linear): 180 degrees
- Three bonding pairs, zero lone pairs (trigonal planar): 120 degrees
- Four bonding pairs, zero lone pairs (tetrahedral): 109.5 degrees
However, the introduction of lone pairs significantly alters these angles. The presence of one or two lone pairs on the central atom leads to a bent molecular geometry, with a bond angle smaller than the ideal angle for the corresponding electron pair arrangement.
Bent Molecules with One Lone Pair (AX₂E)
Molecules with the general formula AX₂E (A = central atom, X = bonding atom, E = lone pair) exhibit a bent shape. A classic example is water (H₂O). Oxygen is the central atom, bonded to two hydrogen atoms and possessing two lone pairs of electrons. The ideal tetrahedral angle (109.5°) is compressed due to the strong repulsion from the lone pairs, resulting in a bond angle of approximately 104.5°.
Bent Molecules with Two Lone Pairs (AX₂E₂)
Molecules with two lone pairs on the central atom (AX₂E₂) also display a bent structure, but with an even smaller bond angle. A prime example is **sulfur dioxide (SO₂) **. The sulfur atom is bonded to two oxygen atoms and has one lone pair. The repulsion from the lone pair compresses the bond angle. Although the electron pair geometry is trigonal planar (120° ideally), the molecular geometry is bent, with a bond angle closer to 119°. Note that the presence of double bonds (one double bond and one single bond in SO2) introduces some complexity which may slightly alter this.
Factors Affecting Bond Angle in Bent Molecules
Several factors influence the precise bond angle in a bent molecule beyond the simple VSEPR model:
-
Electronegativity: The electronegativity difference between the central atom and the surrounding atoms can influence the bond angle. Higher electronegativity differences can lead to a slight increase in bond angle.
-
Hybridization: The hybridization of the central atom also plays a role. Different hybridization states lead to different orbital geometries and therefore different repulsions, influencing the bond angle. For example, sp³ hybridized atoms tend to have smaller bond angles compared to sp² hybridized atoms.
-
Multiple Bonds: The presence of double or triple bonds affects the bond angle. Multiple bonds occupy more space than single bonds due to increased electron density, thus increasing repulsion and potentially altering the bond angle.
-
Steric Effects: Bulky substituents attached to the central atom can cause steric hindrance, slightly altering the bond angle to minimize repulsive interactions between the substituents.
Examples of Bent Molecules
Here are some more examples of bent molecules categorized by the central atom and number of lone pairs:
Group 16 (Chalcogens):
- Water (H₂O): Central atom: Oxygen; Lone pairs: 2; Bond angle: ~104.5°
- Hydrogen sulfide (H₂S): Central atom: Sulfur; Lone pairs: 2; Bond angle: ~92° (Note the significantly smaller angle due to the larger size of sulfur)
- Sulfur dioxide (SO₂): Central atom: Sulfur; Lone pairs: 1; Bond angle: ~119°
Group 15 (Pnictogens):
- Ammonia (NH₃): While not strictly bent (it's trigonal pyramidal), the presence of one lone pair significantly affects the bond angle, leading to a value less than 109.5°.
Group 14 (Tetrels):
- Some molecules involving Group 14 central atoms can also exhibit bent geometries in certain circumstances, especially when the central atom has less than a full octet of electrons.
Beyond VSEPR: More Sophisticated Models
While VSEPR theory provides a useful framework for understanding bent molecules, it's a simplified model. More advanced computational methods, such as density functional theory (DFT), provide more accurate predictions of bond angles by considering electron density distribution and other electronic effects.
Importance of Understanding Bent Molecular Geometry
The bond angle of a bent molecule directly affects its:
- Polarity: Bent molecules are often polar because the bond dipoles do not cancel each other out. This polarity influences physical properties such as boiling point and solubility.
- Reactivity: The spatial arrangement of atoms and lone pairs influences the molecule's ability to interact with other molecules, affecting its reactivity.
- Spectroscopic Properties: The bond angle influences the molecule's vibrational modes, which are detectable through infrared (IR) and Raman spectroscopy.
Conclusion
The bent molecular geometry, arising primarily from the repulsion of lone pairs of electrons, is a common and important structural feature in many molecules. Understanding the factors influencing the bond angle in bent molecules is crucial for predicting their properties and reactivity. While VSEPR theory provides a good starting point, more sophisticated methods are often necessary for accurate predictions. This understanding of molecular geometry forms a fundamental building block for advancing in chemistry and related fields. The diverse examples provided illustrate the wide range of molecules exhibiting this important shape, highlighting the significance of understanding its principles. By considering the various influencing factors discussed, a deeper appreciation for the nuanced world of molecular structures can be gained.
Latest Posts
Latest Posts
-
Which Of The Following Statements Describes The Process Of Globalization
Mar 26, 2025
-
What Are The Greatest Common Factors Of 48
Mar 26, 2025
-
The Unit Of Energy In S I Units Is
Mar 26, 2025
-
How Does Temperature Relate To Kinetic Energy
Mar 26, 2025
-
The Flow Of Electrons Is Called
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Angle Of A Bent Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
