What Is Difference Between Nucleoside And Nucleotide
Juapaving
Mar 20, 2025 · 5 min read
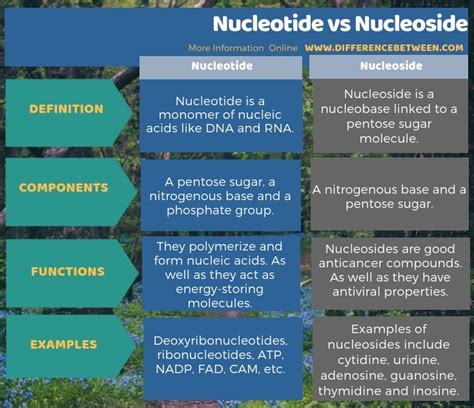
Table of Contents
What's the Difference Between a Nucleoside and a Nucleotide? A Deep Dive into Nucleic Acid Building Blocks
Understanding the fundamental building blocks of life, like nucleic acids, is crucial for comprehending biological processes. Nucleic acids, DNA and RNA, are polymers composed of repeating monomer units. However, these monomers aren't directly nucleotides or nucleosides; they're built from them. This article will delve into the key differences between nucleosides and nucleotides, clarifying their structures, functions, and roles in the broader context of molecular biology.
The Core Distinction: Sugar and Phosphate
The primary difference between a nucleoside and a nucleotide lies in the presence or absence of a phosphate group. This seemingly small difference has significant implications for their biological roles.
-
Nucleoside: A nucleoside is a simple molecule composed of only two components: a nitrogenous base and a five-carbon sugar (pentose). Think of it as the "base unit" – the fundamental structure upon which nucleotides are built.
-
Nucleotide: A nucleotide, in contrast, is a nucleoside with an added phosphate group attached to the sugar molecule. The phosphate group is what fundamentally distinguishes a nucleotide from a nucleoside. This phosphate group is crucial for the formation of phosphodiester bonds that link nucleotides together to create the polymer chains of DNA and RNA.
Components of Nucleosides and Nucleotides: A Detailed Look
Let's examine the constituent parts in more detail:
1. Nitrogenous Bases: The Information Carriers
Both nucleosides and nucleotides contain a nitrogenous base. These bases are crucial for encoding genetic information. There are two main types:
-
Purines: These are double-ringed structures, including adenine (A) and guanine (G). They are larger than pyrimidines.
-
Pyrimidines: These are single-ringed structures, including cytosine (C), thymine (T), and uracil (U). Thymine is found primarily in DNA, while uracil is found primarily in RNA.
2. Pentose Sugars: The Structural Backbone
The pentose sugar in nucleosides and nucleotides provides the structural framework for the molecule. There are two types of pentose sugars:
-
Ribose: This is the sugar found in RNA nucleotides. It has a hydroxyl (-OH) group on the 2' carbon atom.
-
Deoxyribose: This is the sugar found in DNA nucleotides. It lacks the hydroxyl group on the 2' carbon atom; hence, the "deoxy" prefix. This seemingly small difference significantly influences the stability and properties of DNA compared to RNA.
3. Phosphate Group: The Energy Carrier and Linker
The phosphate group (PO₄³⁻) is the key differentiating feature between nucleosides and nucleotides. This group is essential for several reasons:
-
Energy Transfer: ATP (adenosine triphosphate), a nucleotide with three phosphate groups, is the primary energy currency of the cell. The high-energy bonds between the phosphate groups provide the energy needed for many cellular processes.
-
Nucleic Acid Polymerization: The phosphate group facilitates the formation of phosphodiester bonds, which link nucleotides together to create the long chains of DNA and RNA. These bonds connect the 3' carbon of one sugar to the 5' carbon of the next, giving DNA and RNA their characteristic directionality (5' to 3').
Naming Conventions: A Systematic Approach
The naming conventions for nucleosides and nucleotides follow a systematic pattern based on their constituent parts.
Nucleoside Naming:
Nucleosides are named by combining the root name of the base with the suffix "-oside." For example:
- Adenine + ribose = Adenosine
- Guanine + ribose = Guanosine
- Cytosine + ribose = Cytidine
- Thymine + deoxyribose = Thymidine
- Uracil + ribose = Uridine
Nucleotide Naming:
Nucleotide names are more complex because they specify the number of phosphate groups attached. This is typically denoted using prefixes like "mono," "di," or "tri." The base name is included, and the sugar is usually implied unless specifically mentioned as deoxyribose. For example:
- Adenosine monophosphate (AMP) – Adenine + ribose + one phosphate group
- Guanosine diphosphate (GDP) – Guanine + ribose + two phosphate groups
- Cytidine triphosphate (CTP) – Cytosine + ribose + three phosphate groups
- Deoxythymidine triphosphate (dTTP) – Thymine + deoxyribose + three phosphate groups
Biological Significance: Roles in Life Processes
The differences between nucleosides and nucleotides are directly reflected in their biological roles:
Nucleosides: Precursors and Signaling Molecules
While not directly involved in DNA or RNA structure as monomers, nucleosides play crucial supporting roles:
-
Precursors to Nucleotides: Nucleosides are the immediate precursors to nucleotides. They are converted to nucleotides by the addition of a phosphate group via kinase enzymes.
-
Signaling Molecules: Some nucleosides, like adenosine, act as signaling molecules, influencing various cellular processes like neurotransmission and vasodilation.
Nucleotides: Building Blocks of Nucleic Acids and Energy Carriers
Nucleotides have much more diverse and central biological roles compared to nucleosides.
-
DNA and RNA Structure: Nucleotides are the monomers that form the polymeric chains of DNA and RNA. The sequence of nucleotides determines the genetic information encoded within these molecules.
-
Energy Currency: As mentioned previously, ATP is the primary energy currency. Other nucleotides like GTP (guanosine triphosphate) and UTP (uridine triphosphate) also play important roles in energy metabolism and cellular signaling.
-
Coenzymes: Some nucleotides, such as NAD+ (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide), act as coenzymes, participating in crucial metabolic reactions.
-
Second Messengers: Cyclic AMP (cAMP), a cyclic nucleotide derivative, acts as a second messenger in signal transduction pathways.
Summary Table: A Quick Comparison
| Feature | Nucleoside | Nucleotide |
|---|---|---|
| Components | Nitrogenous base + Pentose sugar | Nitrogenous base + Pentose sugar + Phosphate group |
| Phosphate Group | Absent | Present |
| Primary Role | Precursor to nucleotides, signaling | Building block of nucleic acids, energy currency, coenzymes |
| Examples | Adenosine, Guanosine, Cytidine | AMP, ADP, ATP, GTP, CTP, dTTP |
Conclusion: Two Sides of the Same Coin
While nucleosides and nucleotides might seem similar at first glance, their key structural difference – the phosphate group – leads to drastically different biological functions. Nucleosides serve as precursors and signaling molecules, while nucleotides are the fundamental building blocks of nucleic acids and play crucial roles in energy transfer and cellular regulation. Understanding this distinction is crucial for grasping the intricate mechanisms that govern life at the molecular level. Further exploration into the specific roles of different nucleosides and nucleotides within various biological pathways will provide an even deeper understanding of the complexity and elegance of cellular processes.
Latest Posts
Latest Posts
-
The Function Of The Dartos And Cremaster Muscles Is To
Mar 20, 2025
-
What Are The Factors For 74
Mar 20, 2025
-
Relations And Functions Worksheet Grade 11
Mar 20, 2025
-
Difference Between Monohybrid And Dihybrid Inheritance
Mar 20, 2025
-
Common Denominator Of 3 And 7
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is Difference Between Nucleoside And Nucleotide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
