What Is Boiling Point In Kelvin
Juapaving
Apr 07, 2025 · 6 min read
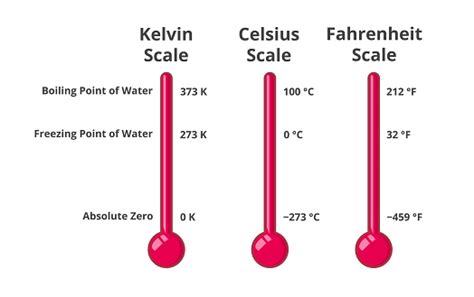
Table of Contents
What is Boiling Point in Kelvin? A Deep Dive into Temperature and Phase Transitions
The boiling point, a fundamental concept in thermodynamics, represents the temperature at which a substance transitions from its liquid phase to its gaseous phase. Understanding the boiling point, particularly when expressed in Kelvin, is crucial in various scientific fields, from chemistry and physics to engineering and meteorology. This comprehensive guide will delve into the intricacies of boiling points, focusing on their representation in the Kelvin scale, and exploring the underlying principles governing this phase transition.
Understanding the Kelvin Scale
Before we delve into boiling points specifically, let's establish a firm understanding of the Kelvin scale. Unlike Celsius and Fahrenheit, which are relative scales with arbitrary zero points, the Kelvin scale is an absolute temperature scale. Its zero point, 0 Kelvin (0 K), represents absolute zero – the theoretically lowest possible temperature where all molecular motion ceases. This absolute nature makes the Kelvin scale incredibly useful in scientific calculations and thermodynamic analyses.
Key Differences from Celsius and Fahrenheit:
- Absolute Zero: The Kelvin scale starts at absolute zero, providing a true measure of thermal energy.
- Unit Size: The size of one Kelvin degree is identical to the size of one Celsius degree. This means a temperature change of 1 K is equivalent to a temperature change of 1 °C.
- No Negative Values: Since Kelvin starts at absolute zero, there are no negative temperatures on this scale.
Converting between scales:
The conversion between Kelvin (K) and Celsius (°C) is straightforward:
- K = °C + 273.15
- °C = K - 273.15
Understanding these conversions is vital when working with boiling points expressed in Kelvin, as data is often presented in Celsius.
The Phenomenon of Boiling
Boiling is a phase transition where a liquid transforms into a gas. This isn't just evaporation – a gradual process occurring at the surface – but rather a rapid vaporization happening throughout the entire liquid volume. Bubbles of vapor form within the liquid and rise to the surface, leading to the characteristic vigorous bubbling associated with boiling.
Factors Affecting Boiling Point:
Several factors influence a substance's boiling point:
- Intermolecular Forces: Stronger intermolecular forces (like hydrogen bonding, dipole-dipole interactions, and London dispersion forces) require more energy to overcome, resulting in higher boiling points. For example, water, with its strong hydrogen bonding, has a relatively high boiling point compared to similar-sized molecules.
- Molecular Weight: Larger molecules generally have higher boiling points due to increased London dispersion forces.
- Pressure: Boiling point is directly related to pressure. At higher pressures, a higher temperature is needed to overcome the external pressure and allow the liquid to boil. This is why pressure cookers cook food faster – the increased pressure elevates the boiling point of water. Conversely, at lower pressures (like at high altitudes), the boiling point decreases.
- Impurities: The presence of dissolved impurities can slightly elevate a liquid's boiling point, a phenomenon known as boiling point elevation.
Boiling Point in Kelvin: Examples and Applications
Now, let's consider several substances and their boiling points in Kelvin:
| Substance | Boiling Point (°C) | Boiling Point (K) |
|---|---|---|
| Water (H₂O) | 100 | 373.15 |
| Ethanol (C₂H₅OH) | 78.3 | 351.45 |
| Methanol (CH₃OH) | 64.7 | 337.85 |
| Oxygen (O₂) | -183 | 90.15 |
| Nitrogen (N₂) | -196 | 77.15 |
| Helium (He) | -268.93 | 4.22 |
These examples illustrate the wide range of boiling points across different substances. The low boiling points of gases like oxygen and nitrogen reflect their weak intermolecular forces. Conversely, water's relatively high boiling point is a testament to the strength of its hydrogen bonds.
Applications of Boiling Point in Kelvin:
The boiling point in Kelvin is crucial in various applications:
- Chemical Processes: Many chemical reactions and processes are conducted at specific temperatures, often involving boiling points. Using Kelvin ensures accurate and consistent results.
- Cryogenics: The study of very low temperatures relies heavily on Kelvin. The boiling points of cryogenic liquids like liquid nitrogen and liquid helium are essential for understanding and controlling cryogenic systems.
- Material Science: The boiling points of materials are critical for designing and manufacturing various products. For example, understanding the boiling point of refrigerants is crucial for designing efficient cooling systems.
- Meteorology: The boiling point of water, as affected by atmospheric pressure, plays a significant role in weather patterns and forecasting.
- Space Exploration: The boiling points of various propellants and cryogenic fuels are critical in spacecraft design and operation.
Understanding Phase Diagrams and the Boiling Point
A phase diagram provides a visual representation of the phases of a substance as a function of temperature and pressure. The boiling point is represented by the line separating the liquid and gas phases. This line isn't always straight; it curves due to the pressure dependence of the boiling point.
The Critical Point:
At a specific temperature and pressure known as the critical point, the distinction between liquid and gas phases disappears. Beyond the critical point, the substance exists as a supercritical fluid, possessing properties of both liquids and gases.
The Triple Point:
The triple point is the unique combination of temperature and pressure where the solid, liquid, and gas phases coexist in equilibrium.
Pressure's Influence on Boiling Point:
The Clausius-Clapeyron equation quantitatively describes the relationship between the vapor pressure of a substance and its temperature, including the boiling point’s pressure dependence. This equation is instrumental in calculating boiling points at various pressures. Understanding this relationship is essential in applications where pressure variations significantly influence the boiling process, such as high-altitude cooking or industrial processes under vacuum conditions.
Advanced Concepts Related to Boiling Point
Beyond the basic understanding of boiling point, several advanced concepts warrant consideration:
- Superheating: A liquid can sometimes be heated above its boiling point without boiling, a phenomenon known as superheating. This typically occurs when there are few nucleation sites (surfaces where bubbles can form).
- Nucleation Sites: Small imperfections or impurities on surfaces in the liquid can act as nucleation sites, facilitating bubble formation and boiling.
- Boiling Point Elevation and Freezing Point Depression: These colligative properties are dependent on the concentration of dissolved solutes and not their identity. Adding solutes elevates boiling points and depresses freezing points.
- Azeotropes: Some liquid mixtures have boiling points that differ significantly from those of their constituent components. These mixtures are called azeotropes and exhibit unique properties during distillation.
Conclusion: The Significance of Boiling Point in Kelvin
The boiling point, expressed in Kelvin, provides a precise and fundamental measure of a substance's thermal behavior. Its accurate determination is crucial in a vast range of scientific, engineering, and industrial applications. Understanding the factors influencing boiling points, coupled with the ability to convert between temperature scales and utilize phase diagrams, is essential for anyone working with materials and processes involving phase transitions. The absolute nature of the Kelvin scale eliminates ambiguity and ensures consistent and reliable calculations, making it the preferred scale in scientific and engineering disciplines dealing with temperature-dependent phenomena. Mastering the concept of boiling point in Kelvin unlocks a deeper understanding of the physical world and its myriad processes.
Latest Posts
Latest Posts
-
2 1 2 As A Percent
Apr 08, 2025
-
Inner Transition Metals In Periodic Table
Apr 08, 2025
-
How Many Feet Is 22 Inches
Apr 08, 2025
-
Things That Start With A Y
Apr 08, 2025
-
Energy Saved Is Energy Produced Assess The Statement
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is Boiling Point In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.