What Is A Pseudo First Order Reaction
Juapaving
Mar 24, 2025 · 6 min read
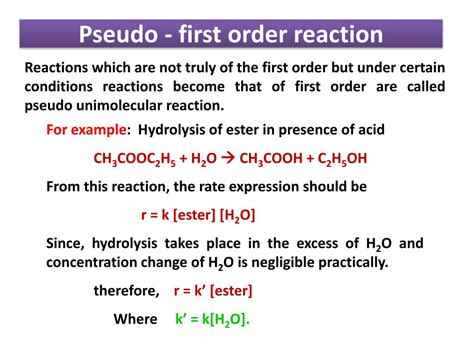
Table of Contents
- What Is A Pseudo First Order Reaction
- Table of Contents
- What is a Pseudo First-Order Reaction? A Comprehensive Guide
- Understanding Reaction Orders
- What is a Pseudo First-Order Reaction?
- The Mechanism: Masking the Higher Order
- Why Use Pseudo First-Order Conditions?
- Mathematical Representation and Analysis
- Examples of Pseudo First-Order Reactions
- 1. Hydrolysis of Esters:
- 2. Enzyme Kinetics (Michaelis-Menten):
- 3. Inversion of Sucrose:
- Determining the True Rate Constant
- Distinguishing Pseudo First-Order from True First-Order
- Applications of Pseudo First-Order Reactions
- Limitations and Considerations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What is a Pseudo First-Order Reaction? A Comprehensive Guide
Understanding reaction kinetics is crucial in chemistry, and within this field, pseudo first-order reactions hold a significant place. This in-depth guide will explore the concept of pseudo first-order reactions, explaining their mechanisms, applications, and importance in various chemical processes. We'll delve into the mathematical representation, providing clear examples and illustrating how to identify and analyze these reactions.
Understanding Reaction Orders
Before diving into pseudo first-order reactions, let's briefly review the concept of reaction order. The order of a reaction describes how the rate of a reaction changes with respect to changes in the concentration of each reactant. This relationship is determined experimentally. For a reaction involving reactants A and B, the general rate law is expressed as:
Rate = k[A]^m[B]^n
where:
- k is the rate constant (specific to the reaction and temperature).
- [A] and [B] represent the concentrations of reactants A and B.
- m and n are the orders of the reaction with respect to A and B, respectively. These are typically integers (0, 1, 2, etc.), but can also be fractional or negative.
- m + n represents the overall order of the reaction.
What is a Pseudo First-Order Reaction?
A pseudo first-order reaction is a second-order (or higher) reaction that is made to behave like a first-order reaction by manipulating the concentrations of the reactants. This manipulation involves having one reactant present in significantly large excess compared to the others. This effectively makes the concentration of the reactant in excess appear constant throughout the reaction.
The Mechanism: Masking the Higher Order
Consider a reaction between two reactants, A and B:
A + B → Products
Let's assume the true rate law is second-order:
Rate = k[A][B]
If we have a large excess of reactant B compared to A (e.g., [B] >> [A]), the concentration of B remains essentially constant during the course of the reaction. Therefore, the term k[B] can be considered a constant:
k' = k[B]
where k' is a new pseudo rate constant. This simplifies the rate law to:
Rate = k'[A]
This equation is characteristic of a first-order reaction, even though the original reaction was second-order. This is the essence of a pseudo first-order reaction – we've created a first-order scenario by controlling the reactant concentrations.
Why Use Pseudo First-Order Conditions?
Creating pseudo first-order conditions offers several advantages:
-
Simplification of Kinetics: First-order reactions are easier to analyze kinetically. Their integrated rate law is simpler, allowing for straightforward determination of the rate constant and half-life.
-
Experimental Convenience: Measuring the concentration of a single reactant is typically easier and less prone to error than monitoring multiple reactants simultaneously.
-
Rate Constant Determination: By performing the reaction under pseudo first-order conditions, we can determine the rate constant (k) even for complex reactions that are difficult to analyze directly.
Mathematical Representation and Analysis
Let's delve deeper into the mathematical aspects of pseudo first-order reactions. The integrated rate law for a first-order reaction is:
ln([A]t) = -kt + ln([A]0)
where:
- [A]t is the concentration of reactant A at time t.
- [A]0 is the initial concentration of reactant A.
- k is the pseudo first-order rate constant (k').
- t is the time.
This equation allows us to plot ln([A]t) versus t, resulting in a straight line with a slope of -k and a y-intercept of ln([A]0). This linear relationship facilitates easy determination of the pseudo first-order rate constant. From this, the actual second-order rate constant (k) can be calculated if the concentration of the reactant in excess ([B]) is known.
Examples of Pseudo First-Order Reactions
Many reactions in chemistry can be studied under pseudo first-order conditions. Here are a few examples:
1. Hydrolysis of Esters:
The acid-catalyzed hydrolysis of an ester is a classic example. The reaction involves the ester and water, but if a large excess of water is used, the reaction becomes pseudo first-order with respect to the ester. The rate law simplifies to:
Rate = k'[ester]
where k' incorporates the concentration of water.
2. Enzyme Kinetics (Michaelis-Menten):
At high substrate concentrations, enzyme-catalyzed reactions can exhibit pseudo first-order kinetics. The rate of the reaction becomes dependent primarily on the enzyme concentration, as the substrate concentration is effectively constant.
3. Inversion of Sucrose:
The acid-catalyzed inversion of sucrose is another example. If a large excess of acid is used, the reaction becomes pseudo first-order with respect to sucrose.
Determining the True Rate Constant
Remember, the pseudo first-order rate constant (k') obtained from experimental data only holds true under the specific conditions of excess reactant concentration. To determine the true rate constant (k) for the higher-order reaction, you must repeat the experiment with varying concentrations of both reactants, keeping neither one in large excess. By analyzing the rate changes with different concentrations of both A and B, one can determine the true reaction orders (m and n) and the true rate constant (k).
Distinguishing Pseudo First-Order from True First-Order
It's crucial to differentiate between a true first-order reaction and a pseudo first-order reaction. A true first-order reaction has a rate that is directly proportional to the concentration of only one reactant, regardless of the concentrations of other reactants. In contrast, a pseudo first-order reaction is fundamentally a higher-order reaction that appears first-order due to the manipulation of reactant concentrations.
Applications of Pseudo First-Order Reactions
Pseudo first-order reactions have numerous applications across diverse fields:
- Chemical Kinetics: Studying reaction mechanisms and determining rate constants.
- Pharmacokinetics: Modeling drug absorption and elimination in the body.
- Environmental Chemistry: Understanding the degradation of pollutants.
- Biochemistry: Analyzing enzyme kinetics and metabolic pathways.
Limitations and Considerations
While pseudo first-order reactions are powerful tools for simplifying kinetic analyses, they have limitations:
-
Assumption of Constant Concentration: The approximation of constant concentration for the reactant in excess may not hold true for all reactions or for prolonged reaction times.
-
Accuracy: The accuracy of the method depends on the degree of excess used, with larger excesses generally yielding more reliable results.
Conclusion
Pseudo first-order reactions offer a powerful technique to simplify complex reaction kinetics by manipulating reactant concentrations. By making one reactant present in significant excess, researchers can reduce the apparent order of the reaction, making the analysis far more straightforward. Understanding the principles of pseudo first-order reactions is essential for effectively studying reaction mechanisms and applying kinetic principles across various scientific disciplines. Remember, though this simplifies analysis, it's crucial to understand the limitations and to determine the true order and rate constant when more complete information is required. The techniques described here are indispensable tools for anyone working with chemical kinetics or related fields.
Latest Posts
Latest Posts
-
Is 6 A Multiple Of 3
Mar 26, 2025
-
What Is Lcm Of 9 And 12
Mar 26, 2025
-
What Is The Factor Of 86
Mar 26, 2025
-
Five Letter Word Beginning With Tho
Mar 26, 2025
-
How Many Suns Can Fit In The Earth
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is A Pseudo First Order Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
