What Is A Positive Ion Called
Juapaving
Mar 21, 2025 · 5 min read
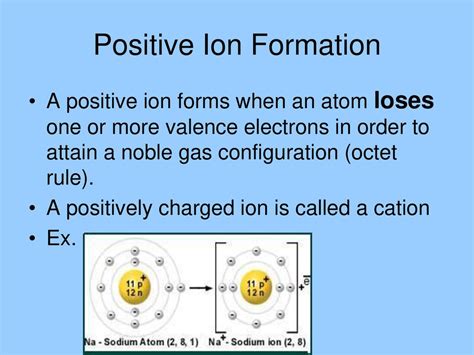
Table of Contents
What is a Positive Ion Called? A Deep Dive into Cations and Their Roles
A positive ion, also known as a cation, is an atom or molecule that has lost one or more electrons. This loss of electrons results in a net positive electrical charge. Understanding cations is fundamental to comprehending various aspects of chemistry, physics, and biology. This article delves into the intricacies of cations, exploring their formation, properties, naming conventions, and diverse roles in numerous scientific fields.
The Formation of Cations: Losing Electrons
The creation of a cation is a process governed by the fundamental principles of atomic structure. Atoms strive for stability, often achieved by attaining a full outermost electron shell (also known as the valence shell). Elements with fewer electrons in their valence shell than needed for a full shell tend to lose those electrons more readily than gaining additional ones. This loss creates a positively charged ion because the number of protons (positively charged particles in the nucleus) now exceeds the number of electrons.
Factors Influencing Cation Formation:
-
Ionization Energy: The energy required to remove an electron from an atom is a crucial factor. Elements with lower ionization energies are more likely to form cations. These elements are generally located on the left side of the periodic table, belonging to groups 1 and 2 (alkali and alkaline earth metals).
-
Electronegativity: Electronegativity measures an atom's ability to attract electrons. Atoms with low electronegativity readily lose electrons, facilitating cation formation.
-
Chemical Reactions: Cations often form during chemical reactions where electron transfer occurs. For instance, in a reaction between sodium (Na) and chlorine (Cl), sodium loses an electron to chlorine, forming a sodium cation (Na⁺) and a chloride anion (Cl⁻).
Naming Cations: A Systematic Approach
Naming cations follows established conventions in chemical nomenclature. Simple cations, formed from single atoms, generally retain the name of the element with the addition of the word "ion" or the charge indicated by Roman numerals in parentheses.
Examples of Simple Cation Naming:
- Sodium ion (Na⁺): Sodium loses one electron to achieve a stable electron configuration.
- Calcium ion (Ca²⁺): Calcium loses two electrons to achieve a stable electron configuration.
- Aluminum ion (Al³⁺): Aluminum loses three electrons to achieve a stable electron configuration.
Transition Metal Cations: Dealing with Variable Charges
Transition metals, located in the middle of the periodic table, can form cations with varying charges. This variability arises because they can lose electrons from multiple electron shells. To distinguish between these different cations, Roman numerals are included in parentheses after the element's name to specify the charge.
Examples of Transition Metal Cation Naming:
- Iron(II) ion (Fe²⁺): Iron has lost two electrons. This is also known as ferrous ion.
- Iron(III) ion (Fe³⁺): Iron has lost three electrons. This is also known as ferric ion.
- Copper(I) ion (Cu⁺): Copper has lost one electron. This is also known as cuprous ion.
- Copper(II) ion (Cu²⁺): Copper has lost two electrons. This is also known as cupric ion.
Properties of Cations: Size, Charge, and Reactivity
The properties of cations are significantly influenced by their charge and size. Generally:
-
Smaller Cations: Have a higher charge density, meaning their positive charge is concentrated in a smaller volume. This leads to stronger interactions with anions (negatively charged ions).
-
Larger Cations: Have lower charge density, resulting in weaker interactions with anions.
-
Charge: The magnitude of the positive charge directly impacts the cation's reactivity and its ability to attract and bind with other ions or molecules. Higher charged cations tend to be more reactive.
The Role of Cations in Various Fields
Cations play crucial roles across diverse scientific disciplines:
1. Biology: Essential for Life
Many cations are essential for biological processes. Examples include:
- Sodium (Na⁺) and Potassium (K⁺): Crucial for nerve impulse transmission and maintaining fluid balance in cells.
- Calcium (Ca²⁺): Essential for muscle contraction, blood clotting, and bone formation.
- Magnesium (Mg²⁺): A cofactor for many enzymes and plays a role in DNA replication and protein synthesis.
2. Chemistry: Driving Chemical Reactions
Cations participate in countless chemical reactions, including:
- Acid-base reactions: Cations can act as acids, donating protons (H⁺) to bases.
- Precipitation reactions: Cations can react with anions to form insoluble salts that precipitate out of solution.
- Redox reactions: Cations can participate in oxidation-reduction reactions, gaining or losing electrons.
3. Materials Science: Building Blocks of Materials
Cations are fundamental components of many materials, shaping their properties:
- Ceramics: Many ceramics are ionic compounds composed of cations and anions, possessing unique properties like hardness, high melting points, and electrical insulation.
- Metals: The metallic bond, which holds metal atoms together, involves a "sea" of delocalized electrons surrounding positively charged metal cations.
4. Geology: Shaping the Earth
Cations are integral to the composition of minerals and rocks. Different types and arrangements of cations contribute to the diverse properties and structures found in geological formations.
Advanced Concepts: Polyatomic Cations and Complex Ions
The world of cations extends beyond simple monatomic ions. Polyatomic cations are positively charged groups of atoms bonded together. These ions often behave as single units in chemical reactions.
Examples of Polyatomic Cations:
- Ammonium ion (NH₄⁺): A common polyatomic cation composed of one nitrogen atom and four hydrogen atoms.
- Hydronium ion (H₃O⁺): Formed when a proton (H⁺) bonds to a water molecule.
Complex ions are formed when a central metal cation is coordinated to surrounding ligands (molecules or ions). These complexes can exhibit unique properties depending on the metal cation and the ligands involved.
Conclusion: The Ubiquitous Nature of Cations
Positive ions, or cations, are fundamental building blocks of matter, playing critical roles in diverse fields. Their formation, properties, and interactions are governed by fundamental chemical and physical principles. Understanding cations is crucial for comprehending various natural processes and for developing new materials and technologies. From the intricate workings of biological systems to the composition of geological formations, cations are ubiquitous and essential to our understanding of the world around us. Their diverse roles continue to be explored and expanded upon as scientific research progresses, highlighting their significance in both fundamental and applied scientific endeavors. The simple concept of a positively charged ion unlocks a vast and fascinating realm of scientific knowledge.
Latest Posts
Latest Posts
-
What Is The S I Unit For Temperature
Mar 27, 2025
-
Write A Short Note On Apiculture
Mar 27, 2025
-
Dissolving Sugar In Water Is A Chemical Change
Mar 27, 2025
-
Which Of The Following Is An Abstract Word
Mar 27, 2025
-
What Type Of Wave Is Water Wave
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is A Positive Ion Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
