What Element Family Does Neon Belong To
Juapaving
Mar 24, 2025 · 7 min read
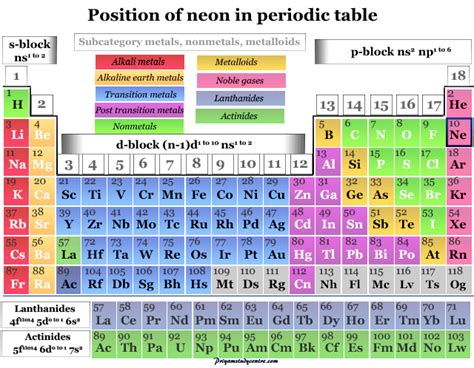
Table of Contents
What Element Family Does Neon Belong To? Exploring the Noble Gases
Neon, that vibrant gas used in dazzling signs, belongs to a fascinating group of elements known as the noble gases (or inert gases). Understanding neon's place within this family reveals crucial insights into its unique properties and behavior. This comprehensive guide delves deep into the noble gas family, focusing on neon's characteristics, applications, and the scientific principles that govern its existence.
Understanding the Noble Gas Family
The noble gases are located in Group 18 (VIIIA) of the periodic table. This group includes helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and the synthetically created oganesson (Og). These elements are characterized by their exceptional stability and low reactivity. This remarkable stability stems from their electronic configuration.
The Significance of Full Valence Shells
Each noble gas atom possesses a complete outermost electron shell, also known as a valence shell. This full valence shell renders them exceptionally unreactive, hence their historical designation as "inert gases." A full valence shell means that the atom has achieved a state of minimum energy, making it highly resistant to forming chemical bonds with other elements. This contrasts sharply with other elements that readily react to gain or lose electrons to achieve a stable electron configuration.
Physical Properties of Noble Gases
Noble gases share several key physical properties:
- Gases at room temperature: All are gases under standard conditions, due to the weak interatomic forces between their atoms.
- Colorless and odorless: In their pure form, they are colorless and odorless, adding to their somewhat mysterious nature.
- Low boiling and melting points: These points increase as you go down the group, reflecting the increasing strength of the weak interatomic forces.
- Poor conductors of electricity (at low pressures): However, they become excellent conductors at high pressures, a property exploited in certain lighting applications.
- Low density: They are significantly less dense than air.
Neon: A Closer Look at a Noble Gas
Neon, with its atomic number 10 and symbol Ne, occupies a prominent place in the noble gas family. Its unique properties, stemming from its electronic configuration, account for its widespread use in various technologies.
Neon's Electronic Configuration
Neon's electronic configuration is 1s²2s²2p⁶. This means it has two electrons in the first energy level (1s²) and eight electrons in the second energy level (2s²2p⁶). The significance lies in the complete filling of the second energy level, achieving the stable octet configuration that is characteristic of noble gases. This complete valence shell is the cornerstone of neon's unreactivity.
Physical and Chemical Properties of Neon
Neon’s specific properties within the noble gas family are:
- Low Reactivity: As expected of a noble gas, neon is extremely unreactive. It rarely forms compounds, and those that have been synthesized exist only under extreme conditions.
- Colorless and Odorless Gas: Like other noble gases, it exists as a colorless, odorless gas at room temperature.
- Low Density: It's lighter than air, making it suitable for specific applications.
- Unique Spectral Lines: Neon is famous for its vibrant red-orange glow when an electric current passes through it, a property exploited in neon signs. This distinctive glow originates from the specific energy transitions of its electrons.
Applications of Neon and Other Noble Gases
The unique properties of noble gases, particularly their stability and inertness, make them valuable in diverse applications:
Neon's Role in Lighting
Neon's most recognizable application is in neon lighting. The characteristic red-orange glow is produced when an electric discharge passes through neon gas under low pressure. While "neon lights" often feature other gases or phosphors to create different colors, the name remains synonymous with this type of vibrant illumination.
Other Noble Gas Applications
Other noble gases also find significant applications:
- Helium (He): Used in balloons, cryogenics (due to its extremely low boiling point), and MRI machines. Its non-reactivity and low density make it ideal for these applications.
- Argon (Ar): Used in welding, as a protective atmosphere in the semiconductor industry, and in incandescent light bulbs. Its inertness prevents oxidation and chemical reactions.
- Krypton (Kr): Used in certain types of lasers and high-intensity lighting.
- Xenon (Xe): Used in high-intensity lamps, flash photography, and medical imaging.
- Radon (Rn): Although radioactive, radon has limited applications in radiation therapy, despite its significant health risks. Its use is carefully controlled due to its radioactivity.
The Discovery and History of Noble Gases
The discovery of noble gases marked a significant advancement in our understanding of the periodic table and chemical bonding:
- Helium's Early Discovery: Helium was initially detected in the sun's spectrum before its terrestrial discovery.
- Argon's Pivotal Role: The discovery of argon, by Lord Rayleigh and Sir William Ramsay, challenged existing chemical theory and paved the way for the recognition of the entire noble gas family. The unexpected observation of an inert gas highlighted gaps in the then-current understanding of the elements.
- Sequential Discoveries: Following argon's discovery, krypton, neon, and xenon were identified through fractional distillation of liquid air. Radon, a radioactive noble gas, was later discovered as a decay product of radium.
- Oganesson's Synthetic Creation: Oganesson, the newest member of the noble gas family, is a synthetically created element with an extremely short half-life, further highlighting the ongoing expansion of our understanding of the periodic table.
Neon's Unique Spectral Lines and Atomic Structure
Neon's distinctive red-orange glow in neon signs arises from its unique atomic structure and the interaction of its electrons with light:
- Energy Levels and Electron Transitions: When an electric current passes through neon gas, electrons in the neon atoms absorb energy and jump to higher energy levels. When these excited electrons return to their lower energy levels, they emit light of specific wavelengths, corresponding to the energy differences between the levels.
- Spectral Fingerprints: The specific wavelengths of light emitted by neon create its unique "spectral fingerprint," a characteristic pattern that distinguishes it from other elements. This principle is central to spectroscopy, a powerful analytical technique.
- Color Variation in Neon Signs: While pure neon produces a characteristic red-orange glow, other colors can be achieved by mixing neon with other gases or by coating the inside of the tube with phosphors. The phosphors absorb the neon's light and re-emit it at different wavelengths, creating a wider range of colors.
The Significance of Neon and its Family in Scientific Advancements
The noble gases, including neon, have played a significant role in advancing scientific understanding:
- Development of Atomic Theory: The unique behavior of noble gases helped refine our understanding of atomic structure and electron configuration, leading to the development of the modern periodic table. Their inertness provided crucial evidence for the stability associated with full valence shells.
- Advancements in Spectroscopy: The spectral lines of noble gases, including neon's distinct red-orange glow, played a crucial role in the development of spectroscopy, a fundamental technique for identifying elements and studying their properties.
- Technological Innovations: The inertness and other unique properties of noble gases have enabled countless technological advancements, ranging from lighting and welding to medical imaging and cryogenics.
Conclusion: Neon's Essential Role in the Noble Gas Family
Neon's membership in the noble gas family is fundamentally linked to its electronic configuration, which bestows upon it its characteristic inertness and unique spectral properties. This unique combination is responsible for its widespread applications in lighting, contributing to its prominent position within this remarkable family of elements. Understanding neon's place within the noble gas family provides a fascinating glimpse into the principles that govern the behavior of matter at an atomic level, and highlights the crucial role these elements play in scientific understanding and technological advancement. From the vibrant glow of neon signs to the diverse applications of other noble gases, their contributions to our world are undeniable.
Latest Posts
Latest Posts
-
Least Common Multiple Of 25 And 15
Mar 26, 2025
-
What Is The Lcm Of 18 And 6
Mar 26, 2025
-
How Many Mm In 5 Cm
Mar 26, 2025
-
What Is The Simplest Form Of 6 12
Mar 26, 2025
-
How Many Chambers Of Heart In Fish
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Element Family Does Neon Belong To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
